- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 44(4); 2020 > Article
-
ReviewGuideline/Fact Sheet Sodium-Glucose Cotransporter-2 Inhibitor for Renal Function Preservation in Patients with Type 2 Diabetes Mellitus: A Korean Diabetes Association and Korean Society of Nephrology Consensus Statement
-
Tae Jung Oh1,2
 , Ju-Young Moon3
, Ju-Young Moon3 , Kyu Yeon Hur4, Seung Hyun Ko5, Hyun Jung Kim6, Taehee Kim7, Dong Won Lee8
, Kyu Yeon Hur4, Seung Hyun Ko5, Hyun Jung Kim6, Taehee Kim7, Dong Won Lee8 , Min Kyong Moon1,9
, Min Kyong Moon1,9 , The Committee of Clinical Practice Guideline, Korean Diabetes Association, Committee of the Cooperative Studies, Korean Society of Nephrology
, The Committee of Clinical Practice Guideline, Korean Diabetes Association, Committee of the Cooperative Studies, Korean Society of Nephrology -
Diabetes & Metabolism Journal 2020;44(4):489-497.
DOI: https://doi.org/10.4093/dmj.2020.0172
Published online: August 21, 2020
1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
3Division of Nephrology, Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, Korea.
4Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
5Division of Endocrinology and Metabolism, Department of Internal Medicine, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea.
6Department of Preventive Medicine, Korea University College of Medicine, Seoul, Korea.
7Division of Nephrology, Department of Internal Medicine, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
8Division of Nephrology, Department of Internal Medicine, Pusan National University School of Medicine, Yangsan, Korea.
9Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea.
- Corresponding author: Dong Won Lee. Division of Nephrology, Department of Internal Medicine, Pusan National University School of Medicine, 20 Geumo-ro, Mulgeum-eup, Yangsan 50612, Korea. dongwonlee@pusan.ac.kr
- Corresponding author: Min Kyong Moon. Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University College of Medicine, 20 Boramae-ro 5-gil, Dongjak-gu, Seoul 07061, Korea. mkmoon@snu.ac.kr
- *Tae Jung Oh and Ju-Young Moon contributed equally to this study as first authors.
Copyright © 2020 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- ABSTRACT
- INTRODUCTION
- RECENT RECOMMENDATIONS REGARDING SGLT2 INHIBITOR USE IN PATIENTS WITH T2DM
- EFFECTS OF SGLT2 INHIBITOR ON RENAL FUNCTION
- KOREAN DIABETES ASSOCIATION AND KOREAN SOCIETY OF NEPHROLOGY JOINT CONSENSUS STATEMENTS ON THE USE OF SGLT2 INHIBITOR IN T2DM FOR RENAL FUNCTION PRESERVATION
- ACKNOWLEDGMENTS
- NOTES
- SUPPLEMENTARY MATERIALS
- REFERENCES
ABSTRACT
- Diabetes is a leading cause of end-stage renal disease. Therefore, prevention of renal dysfunction is an important treatment goal in the management of diabetes. The data of landmark cardiovascular outcome trials of sodium-glucose cotransporter-2 (SGLT2) inhibitor showed profound reno-protective effects. The Korean Diabetes Association and the Korean Society of Nephrology reviewed clinical trials and performed meta-analysis to assess the effects of SGLT2 inhibitors on the preservation of estimated glomerular filtration rate (eGFR). We limited the data of SGLT2 inhibitors which can be prescribed in Korea. Both eGFR value and its change from the baseline were significantly more preserved in the SGLT2 inhibitor treatment group compared to the control group after 156 weeks. However, some known adverse events were increased in SGLT2 inhibitor treatment, such as genital infection, diabetic ketoacidosis, and volume depletion. We recommend the long-term use SGLT2 inhibitor in patients with type 2 diabetes mellitus (T2DM) for attenuation of renal function decline. However, we cannot generalize our recommendation due to lack of long-term clinical trials testing reno-protective effects of every SGLT2 inhibitor in a broad range of patients with T2DM. This recommendation can be revised and updated after publication of several large-scale renal outcome trials.
- Diabetic kidney disease (DKD) is a global problem, and the prevalence and incidence are increasing strikingly. Along with the accelerating incidence of DKD, the total number of patients with end-stage renal disease (ESRD) undergoing maintenance dialysis has grown by approximately 7% to 10% per year in Korea [123]. DKD is the most prevalent cause of ESRD (50.2% of new ESRD patients in 2016) in Korea from 1994 [4]. All current efforts for type 2 diabetes mellitus (T2DM) are devoted to the control of hyperglycemia to prevent the development of micro- and macrovascular complications. The cornerstone of therapy to prevent DKD is the strict control of blood pressure with the renin-angiotensin-aldosterone system (RAAS) blockade and blood glucose levels [5]. However, many patients with diabetes progress to chronic kidney disease (CKD) despite standard treatment. These kinds of patients usually have profound amounts of albuminuria despite the use of RAAS blocking agents, and renal function declines rapidly. Furthermore, reduced estimated glomerular filtration rate (eGFR) is independently associated with all-cause mortality and cardiovascular disease [6]. There is an unmet clinical need for diabetes treatment to prevent or delay DKD progression.
- Sodium-glucose cotransporter-2 (SGLT2) inhibitor is an emerging antidiabetic medication, and its cardio-protective effect has been proven from the large-scale cardiovascular outcome trials of Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes (EMPA-REG) OUTCOME [7], Canagliflozin Cardiovascular Assessment Study (CANVAS) Program [8], and Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) [9]. In these studies, renal outcome was analyzed as a secondary outcome. Empagliflozin showed 49% reduction of incident or worsening nephropathy (progression to macroalbuminuria, doubling of the serum creatinine level, initiation of renal-replacement therapy, or death from renal disease) [10], and dapagliflozin reduced 47% of renal-specific outcomes (a sustained decline of at least 40% in eGFR to less than 60 mL/min/1.73 m2, ESRD, or death from renal or cardiovascular causes) [11]. The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial [12] was the first trial to show its reno-protective effects as a primary outcome from a large-scale randomized clinical trial (among 4,401 subjects with T2DM). However, canagliflozin is not available in Korea. Therefore, it is necessary to investigate the renal benefits of SGLT2 inhibitors, which can be prescribed in Korea [1314]. In real clinical practice, we monitored patient's renal function using eGFR as described in other clinical trials assessed it. Therefore, among various renal outcomes, we aimed to analyze the eGFR after long-term treatment of SGLT2 inhibitors.
INTRODUCTION
- In August 2019, the American Diabetes Association revised the online version of the Standards of Medical Care in Diabetes, adopting the results of the CREDENCE trial [15]. It recommended to consider SGLT2 inhibitor treatment in patients with T2DM and CKD (level of evidence C). After that, it updated the recommendation level to level of evidence A and specified the indication of SGLT2 inhibitor, for patients with T2DM and DKD with eGFR ≥30 mL/min/1.73 m2 and albuminuria in 2020 [16]. Diabetes Canada also recommended SGLT2 inhibitor in T2DM with clinical cardiovascular disease and with eGFR ≥30 mL/min/1.73 m2 to reduce the risk of progression of CKD (Grade B, level 2 for empagliflozin and Grade C, level 3 for canagliflozin) in 2018 [17]. The Committee of Clinical Practice Guideline of the Korean Diabetes Association updated the 6th Clinical Practice Guideline in 2019. In this guideline, SGLT2 inhibitor was recommended as a second-line drug, and the committee emphasized its cardioprotective effect [18]. However, it did not yet contain renal outcomes of SGLT2 inhibitor. In this statement, we firstly announced the reno-protective effects of SGLT2 inhibitor considering the situation of Korea.
RECENT RECOMMENDATIONS REGARDING SGLT2 INHIBITOR USE IN PATIENTS WITH T2DM
- Results of cardiovascular outcome trials using SGLT2 inhibitors
- Neuen et al. [19] reported the meta-analysis data of EMPA-REG Outcome, CANVAS Program, CREDENCE, and DECLARE-TIMI 58. This meta-analysis demonstrated that SGLT2 inhibitors reduced the risk of ESRD and death due to kidney disease (relative risk of 0.67). This beneficial effect has been shown consistently across baseline eGFR levels in those studies. However, a meta-analysis result excluding CREDENCE trial showed that the reno-protective effects were attenuated in patients with more advanced renal dysfunction at baseline eGFR below 60 mL/min/1.73 m2 [20]. The inclusion and exclusion criteria of the original randomized clinical trials were not the same. For example, the EMPA-REG trial [10] included subjects with their eGFR of at least 30 mL/min/1.73 m2, in contrast DECLARE-TIMI 58 study [11] included subjects whose eGFR were equal to or higher than 60 mL/min/1.73 m2. In this regard, 25.5% of subjects in the EMPA-REG study and only 7.4% of participants in DECALRE-TIMI 58 had eGFR <60 mL/min/1.73 m2. Furthermore, a large number of participants were not followed up, and Asian patients made up the minority of participants in each study, 21.6% in the EMPA-REG study and 13.4% in the DECLARE-TIMI 58 study. For this reason, uncertainty remains about the reno-protective effect of SGLT2 inhibitor in Asian populations with a broad range of renal function, especially in subjects with reduced renal function. In this study, we reviewed current evidence considering patients' baseline renal function and performed meta-analysis to determine whether SGLT2 inhibitor can be recommended to Korean subjects with T2DM.
- Meta-analysis of large clinical trials using SGLT2 inhibitors
- We searched PubMed, Embase, and Cochrane Central Register of Controlled Library up to March 2020, using search terms including dapagliflozin, empagliflozin, ipragliflozin, and ertugliflozin, which are drugs available in Korea. We included long-term large-scale randomized placebo controlled trials whose treatment duration was at least 52 weeks in T2DM with more than 100 participants in total. No studies on ipragliflozin and ertugliflozin met these criteria. Finally, we included 12 articles for 11 clinical studies (Supplementary Table 1) [91021222324252627282930]. In this meta-analysis, final eGFR level or delta eGFR from baseline was compared between SGLT2 inhibitor treatment group and placebo-control according to the method presented in the original studies, and we analyzed the data of low and high doses of empagliflozin separately.
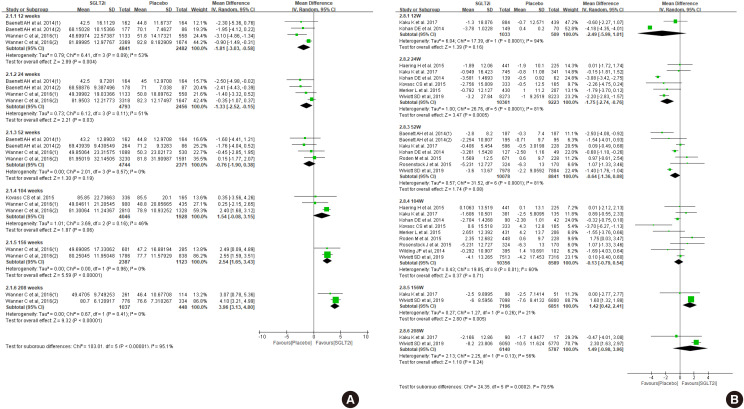
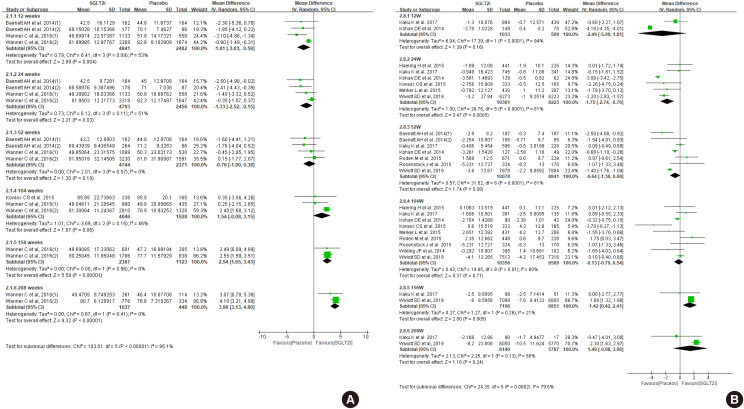
- First, we compared the eGFR levels at each time point between SGLT2 inhibitor and control. As shown in Fig. 1A, SGLT2 inhibitor treatment showed a lower eGFR level than the control at 12 weeks, −1.81 mL/min/1.73 m2 (95% confidence interval [CI], −3.03 to −0.58) and at 24 weeks, −1.33 mL/min/1.73 m2 (95% CI, −2.52 to −0.15). However, a favorable effect on eGFR was observed after at least 156 weeks of treatment. At 208 weeks of treatment, the mean difference of eGFR was 3.96 mL/min/1.73 m2 (95% CI, 3.13 to 4.80) between groups. In general, the improving trend of eGFR was observed in the SGLT2 inhibitor group as the treatment duration was prolonged. In terms of eGFR change from baseline, mean difference was 1.42 mL/min/1.73 m2 (95% CI, 0.42 to 2.41) at 156 weeks (Fig. 1B). Therefore, SGLT2 inhibitor treatment showed reno-protective effects after long-term treatment.
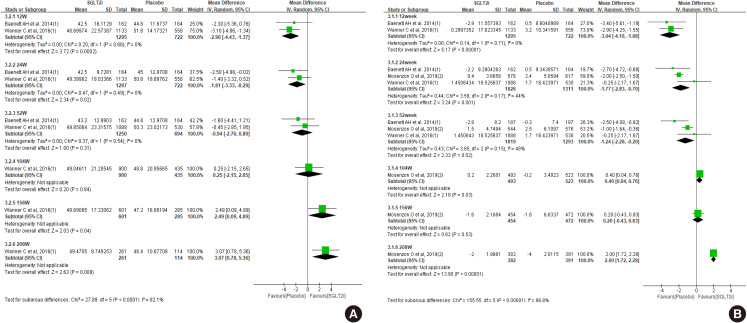
- The glucose lowering effect of SGLT2 inhibitors was consistently observed: the mean difference in decline of glycosylated hemoglobin (HbA1c) was −0.54 (95% CI, −0.67 to −0.41) at 52 weeks and −0.62 (95% CI, −0.75 to −0.48) at 104 weeks (Fig. 2A). The magnitude of body weight reduction was also greater in SGLT2 inhibitor treatment than control treatment (Fig. 2B). Both systolic and diastolic blood pressure were more decreased in SGLT2 inhibitor treatment (Fig. 2C and D).
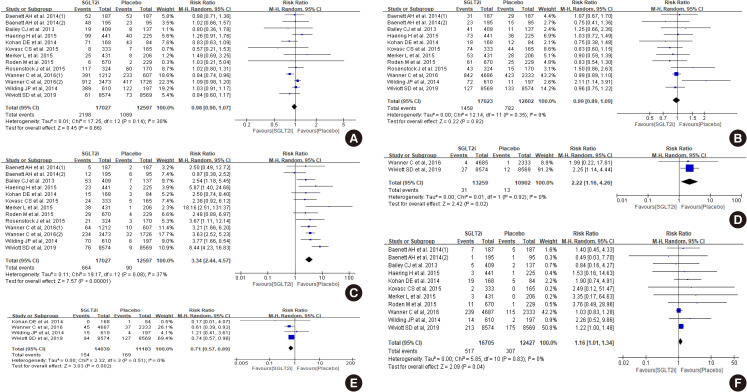
- There was no difference between SGLT2 inhibitor and control groups in hypoglycemia events and urinary tract infection (Fig. 3A and B). However, more subjects were diagnosed with genital infection (risk ratio of 3.34) (Fig. 3C) and diabetic ketoacidosis (risk ratio 2.22) (Fig. 3D). Acute kidney injury was less in SGLT2 inhibitor compared to control (risk ratio 0.71) (Fig. 3E), but volume depletion was slightly more common in the SGLT2 inhibitor group (risk ratio 1.16) (Fig. 3F).
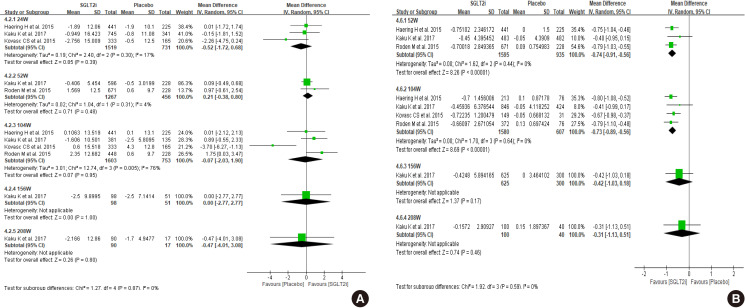
- The clinical studies we included for this systemic review and meta-analysis did not enroll a sufficient number of patients with an eGFR below 60 mL/min/1.73 m2. The patients with eGFR below 60 mL/min/1.73 m2 included 7.4% of participants in the dapagliflozin study (DECLARE-TIMI 58) and 25.5% of participants in the empagliflozin study (EMPA-REG). Dapagliflozin did not demonstrate the prevention of eGFR decline in patients with an eGFR below 60 mL/min/1.73 m2 compared to placebo group during 4-year follow-up (P=0.053) [11]. This negative result for preventing the eGFR decline with dapagliflozin could be due to an insufficient number of enrolled patients with an eGFR below 60 mL/min/1.73 m2 since dapagliflozin reduced the rate of eGFR decline in patients with an eGFR above 60 mL/min/1.73 m2. In the empagliflozin study, patients with eGFR below 60 mL/min/1.73 m2 treated with the empagliflozin showed higher eGFR compared to the placebo group from 156 weeks, while the analysis with total enrolled patients showed higher eGFR at 104 weeks (Fig. 4A vs. Fig. 1A). In terms of eGFR change from baseline, SGLT2 inhibitors showed beneficial effect at 208-week treatment compared to control (Fig. 4B). Both SGLT2 inhibitor studies revealed the initial decrease of the eGFR after SGLT2 inhibitors treatment since the mechanism of SGLT2 inhibitors may be due to relieving vasodilation of the afferent arteriole and following decrease of glomerular hyperfiltration in diabetes [31]. This initial decrease of the eGFR after SGLT2 inhibitor treatment was more significant in patients with an eGFR below 60 mL/min/1.73 m2.
- The risk of a sustained decrease in eGFR by at least 40% to less than 60 mL/min/1.73 m2, ESRD, or renal death was lower in the dapagliflozin group than those in the placebo group, but there was no statistical significance in patients with eGFR below 60 mL/min/1.73 m2 (hazard ratio, 0.60; 95% CI, 0.35 to 1.02; P=0.059) [11]. In the EMPA-REG study, incident or worsening nephropathy was significantly lower in the empagliflozin group with eGFR below 60 mL/min per 1.73 m2 than in the placebo group (hazard ratio, 0.58; 95% CI, 0.47 to 0.71; P<0.001) [10]. The adverse events in the EMPA-REG study were similar between the empagliflozin group and the placebo group in patients with an eGFR of 60 mL/min/1.73 m2 or more and in patients with an eGFR of 59 mL/min/1.73 m2 or less. In our meta-analysis, patients with eGFR <60 mL/min/1.73 m2 showed less hypoglycemia and more genital infection in SGLT2 inhibitor treatment compared to control (Supplementary Fig. 1).
- We arbitrarily defined Asian-dominant study when Asians made up more than 40% of total subjects. Kaku et al. [21] analyzed the subgroup data of the Asian population from the EMPA-REG OUTCOME trial, which was the longest and largest trial in our meta-analysis. Asian-dominant studies showed no significant difference in eGFR change between groups (Fig. 5A). Only one study was analyzed after 156-week treatment in this meta-analysis; therefore, it is not conclusive whether Asians may experience similar benefits of renal preservation in long-term treatment of SGLT2 inhibitor compared to the non-Asian population. Other parameters, such as HbA1c, body weight, blood pressure, and adverse events were similar with meta-analysis of total population (Fig. 5B, Supplementary Fig. 2).
EFFECTS OF SGLT2 INHIBITOR ON RENAL FUNCTION
Search strategy and selection criteria
Effect of SGLT2 inhibitor on eGFR
Effect of SGLT2 inhibitor on glycosylated hemoglobin, body weight, and blood pressure
Adverse effects of SGLT2 inhibitor
Effects of SGLT2 inhibitor on renal function among patients with eGFR below 60 mL/min/1.73 m2
Results of Asian dominant study
- Long-term treatment of SGLT2 inhibitor has a preventive effect on decline of renal function in some patients with T2DM; therefore, long-term treatment of SGLT2 inhibitor is recommended under continuous monitoring of renal function (eGFR) (weak recommendation, low quality of evidence).
- Strength of the recommendation
- Study participants were mainly from Western countries. Their baseline body mass index was near 30 kg/m2, which is relatively higher than Korean subjects with T2DM. Furthermore, Asian dominant studies are lacking, and meta-analysis including only Asian dominant studies did not show statistically significant effects on renal preservation. In addition, only two studies included subjects whose eGFR was less than 60 mL/min/1.73 m2 in this analysis. The immediate decline of renal function was frequently observed in subjects with eGFR <60 mL/min/1.73 m2; therefore, more attention should be focused on this population. In this regards, we cannot recommend the use of SGLT2 inhibitors to all of the patients with T2DM. We need further studies aiming to see renal effects as a primary outcome and including a meaningful number of Asian patients and subjects with a broad range of renal function. The Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) trial (NCT03036150) and EMPA-KIDNEY study (NCT03594110) will provide evidence in this field. This recommendation can be revised and updated after publication of these two landmark studies.
- Quality of evidence
- Authors independently assessed risk of bias at the study level using the revised Cochrane risk of bias tool for randomized trials (risk of bias 2.0), and any disagreements were resolved by consensus among authors. Risk of bias was generally low except two studies that had high risk of bias due to missing outcome data (Supplementary Fig. 3). A large number of patients were not followed up at final assessment. More than 50% of participants were not followed up in three studies. We do not know distinct clinical characteristics between subjects who were followed up successfully and those who were not. Therefore, there is uncertainty due to high dropout rate.
- Other consideration
- The mainstay of prevention and treatment for CKD in T2DM included optimal treatment of hyperglycemia, dyslipidemia, obesity, and blood pressure using RAAS blockade. These general recommendations should be followed, and also consider patient's preference regarding the benefits of SGLT2 inhibitors and the possible disadvantages, such as urinary frequency, unwanted body weight loss, genital infection, and cost. We should educate patients for preventing volume depletion and genital infection. From the physicians' perspectives, immediate decline of renal function is concerned, and a follow-up plan for monitoring eGFR must be developed.
KOREAN DIABETES ASSOCIATION AND KOREAN SOCIETY OF NEPHROLOGY JOINT CONSENSUS STATEMENTS ON THE USE OF SGLT2 INHIBITOR IN T2DM FOR RENAL FUNCTION PRESERVATION
-
Acknowledgements
- This work was performed through the cooperation of the Korean Diabetes Association and the Korean Society of Nephrology.
ACKNOWLEDGMENTS
-
This manuscript is simultaneously published in The Korean Journal of Medicine, Diabetes & Metabolism Journal, Kidney Research and Clinical Practice, and The Journal of Korean Diabetes.
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
NOTES
SUPPLEMENTARY MATERIALS
Supplementary Table 1
Supplementary Fig. 1
Supplementary Fig. 2
- 1. Jin DC, Yun SR, Lee SW, Han SW, Kim W, Park J. Current characteristics of dialysis therapy in Korea: 2015 registry data focusing on elderly patients. Kidney Res Clin Pract 2016;35:204-211. ArticlePubMedPMC
- 2. Jin DC. Major changes and improvements of dialysis therapy in Korea: review of end-stage renal disease registry. Korean J Intern Med 2015;30:17-22. ArticlePubMedPDF
- 3. Jin DC, Yun SR, Lee SW, Han SW, Kim W, Park J, Kim YK. Lessons from 30 years' data of Korean end-stage renal disease registry, 1985–2015. Kidney Res Clin Pract 2015;34:132-139. ArticlePubMedPMC
- 4. Jin DC, Yun SR, Lee SW, Han SW, Kim W, Park J, Kim YK. Current characteristics of dialysis therapy in Korea: 2016 registry data focusing on diabetic patients. Kidney Res Clin Pract 2018;37:20-29. ArticlePubMedPMCPDF
- 5. American Diabetes Association. 10. Microvascular complications and foot care: standards of medical care in diabetes-2018. Diabetes Care 2018;41:S105-S118. ArticlePubMedPDF
- 6. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-1305. ArticlePubMed
- 7. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117-2128. ArticlePubMed
- 8. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644-657. ArticlePubMed
- 9. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS. DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347-357. ArticlePubMed
- 10. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B. EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323-334. ArticlePubMed
- 11. Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Heerspink HJL, Zelniker TA, Dwyer JP, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Kato ET, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Raz I. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019;7:606-617. ArticlePubMed
- 12. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW. CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295-2306. ArticlePubMed
- 13. Lee JY, Cho Y, Lee M, Kim YJ, Lee YH, Lee BW, Cha BS, Kang ES. Predictors of the therapeutic efficacy and consideration of the best combination therapy of sodium-glucose co-transporter 2 inhibitors. Diabetes Metab J 2019;43:158-173. ArticlePubMedPMCPDF
- 14. Hong AR, Koo BK, Kim SW, Yi KH, Moon MK. Efficacy and safety of sodium-glucose cotransporter-2 inhibitors in Korean patients with type 2 diabetes mellitus in real-world clinical practice. Diabetes Metab J 2019;43:590-606. ArticlePubMedPMCPDF
- 15. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2019. Diabetes Care 2019;42:S124-S138. ArticlePubMedPDF
- 16. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2020. Diabetes Care 2020;43:S135-S151. ArticlePubMedPDF
- 17. Diabetes Canada Clinical Practice Guidelines Expert Committee. McFarlane P, Cherney D, Gilbert RE, Senior P. Chronic kidney disease in diabetes. Can J Diabetes 2018;42 Suppl 1:S201-S209. ArticlePubMed
- 18. Kim MK, Ko SH, Kim BY, Kang ES, Noh J, Kim SK, Park SO, Hur KY, Chon S, Moon MK, Kim NH, Kim SY, Rhee SY, Lee KW, Kim JH, Rhee EJ, Chun S, Yu SH, Kim DJ, Kwon HS, Park KS. Committee of Clinical Practice Guidelines, Korean Diabetes Association. 2019 Clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J 2019;43:398-406. ArticlePubMedPMCPDF
- 19. Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, Bompoint S, Levin A, Jardine MJ. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019;7:845-854. ArticlePubMed
- 20. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31-39. ArticlePubMed
- 21. Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ. EMPA-REG OUTCOME®. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from EMPA-REG OUTCOME. Circ J 2017;81:227-234. ArticlePubMed
- 22. Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC. EMPA-REG RENAL trial investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2014;2:369-384. ArticlePubMed
- 23. Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med 2013;11:43. ArticlePubMedPMCPDF
- 24. Haering HU, Merker L, Christiansen AV, Roux F, Salsali A, Kim G, Meinicke T, Woerle HJ, Broedl UC. EMPA-REG EXTEND METSU investigators. Empagliflozin as add-on to metformin plus sulphonylurea in patients with type 2 diabetes. Diabetes Res Clin Pract 2015;110:82-90. ArticlePubMed
- 25. Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014;85:962-971. ArticlePubMed
- 26. Kovacs CS, Seshiah V, Merker L, Christiansen AV, Roux F, Salsali A, Kim G, Stella P, Woerle HJ, Broedl UC. on behalf of the EMPA-REG EXTEN PIO investigators.Empagliflozin as add-on therapy to pioglitazone with or without metformin in patients with type 2 diabetes mellitus. Clin Ther 2015;37:1773-1788. ArticlePubMed
- 27. Merker L, Haring HU, Christiansen AV, Roux F, Salsali A, Kim G, Meinicke T, Woerle HJ, Broedl UC. EMPA-REG EXTEND MET investigators. Empagliflozin as add-on to metformin in people with type 2 diabetes. Diabet Med 2015;32:1555-1567. ArticlePubMed
- 28. Roden M, Merker L, Christiansen AV, Roux F, Salsali A, Kim G, Stella P, Woerle HJ, Broedl UC. EMPA-REG EXTEND MONO investigators. Safety, tolerability and effects on cardiometabolic risk factors of empagliflozin monotherapy in drug-naïve patients with type 2 diabetes: a double-blind extension of a phase III randomized controlled trial. Cardiovasc Diabetol 2015;14:154. ArticlePubMedPMC
- 29. Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ. EMPA-REG BASALTM trial investigators. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 2015;17:936-948. ArticlePubMedPMCPDF
- 30. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S. Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab 2014;16:124-136. ArticlePubMed
- 31. Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. SGLT2 inhibitors and the diabetic kidney. Diabetes Care 2016;39 Suppl 2:S165-S171. ArticlePubMedPDF
REFERENCES
Effect of sodium-glucose cotransporter-2 (SGLT2) inhibitors on (A) estimated glomerular filtration rate (eGFR), and (B) change of eGFR from baseline. SD, standard deviation; IV, inverse variance; CI, confidence interval.
Effect of sodium-glucose cotransporter-2 (SGLT2) inhibitors on change of (A) glycosylated hemoglobin, (B) body weight, (C) systolic blood pressure, and (D) diastolic blood pressure. SD, standard deviation; IV, inverse variance; CI, confidence interval.

Effect of sodium-glucose cotransporter-2 (SGLT2) inhibitors on (A) hypoglycemia, (B) urinary tract infection, (C) genital infection, (D) diabetic ketoacidosis, (E) acute kidney injury, and (F) volume depletion. M-H, Mantel-Haenszel; CI, confidence interval.

Figure & Data
References
Citations

- Real-World Treatment Patterns according to Clinical Practice Guidelines in Patients with Type 2 Diabetes Mellitus and Established Cardiovascular Disease in Korea: Multicenter, Retrospective, Observational Study
Ye Seul Yang, Nam Hoon Kim, Jong Ha Baek, Seung-Hyun Ko, Jang Won Son, Seung-Hwan Lee, Sang Youl Rhee, Soo-Kyung Kim, Tae Seo Sohn, Ji Eun Jun, In-Kyung Jeong, Chong Hwa Kim, Keeho Song, Eun-Jung Rhee, Junghyun Noh, Kyu Yeon Hur
Diabetes & Metabolism Journal.2024; 48(2): 279. CrossRef - Renoprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors: Focusing on Renal Hemodynamics
Nam Hoon Kim, Nan Hee Kim
Diabetes & Metabolism Journal.2022; 46(4): 543. CrossRef - Real-World Prescription Patterns and Barriers Related to the Use of Sodium-Glucose Cotransporter 2 Inhibitors among Korean Patients with Type 2 Diabetes Mellitus and Cardiovascular Disease
Jong Ha Baek, Ye Seul Yang, Seung-Hyun Ko, Kyung Do Han, Jae Hyeon Kim, Min Kyong Moon, Jong Suk Park, Byung-Wan Lee, Tae Jung Oh, Suk Chon, Jong Han Choi, Kyu Yeon Hur
Diabetes & Metabolism Journal.2022; 46(5): 701. CrossRef
- Figure
- Related articles
-
- Evaluation and Management of Patients with Diabetes and Heart Failure: A Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement
- Lipid Management in Korean People with Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
- Comprehensive Understanding for Application in Korean Patients with Type 2 Diabetes Mellitus of the Consensus Statement on Carbohydrate-Restricted Diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension
- Comparative Effects of Sodium-Glucose Cotransporter 2 Inhibitor and Thiazolidinedione Treatment on Risk of Stroke among Patients with Type 2 Diabetes Mellitus

 KDA
KDA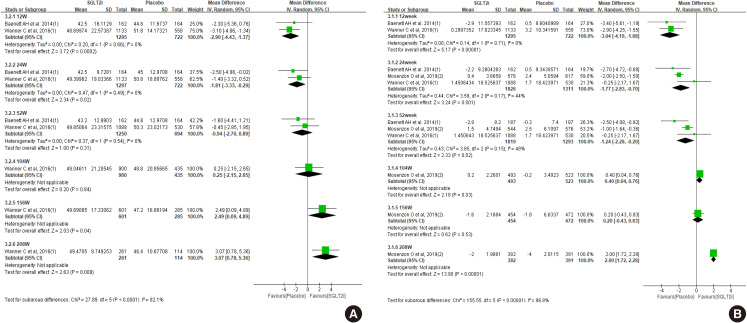
 PubReader
PubReader ePub Link
ePub Link Cite
Cite