
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Article by topic
- Page Path
- HOME > Browse > Article by topic
Reviews
- Pathophysiology
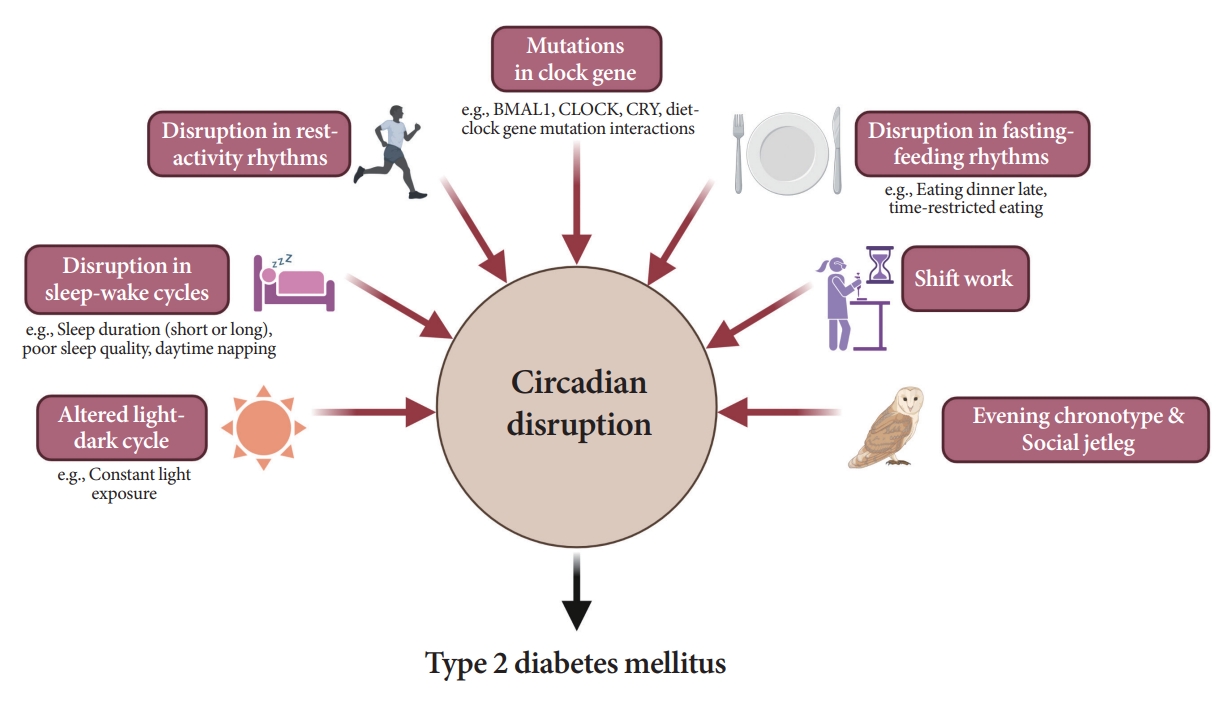
- Attention to Innate Circadian Rhythm and the Impact of Its Disruption on Diabetes
- Da Young Lee, Inha Jung, So Young Park, Ji Hee Yu, Ji A Seo, Kyeong Jin Kim, Nam Hoon Kim, Hye Jin Yoo, Sin Gon Kim, Kyung Mook Choi, Sei Hyun Baik, Nan Hee Kim
- Diabetes Metab J. 2024;48(1):37-52. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0193
- 2,027 View
- 214 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Novel strategies are required to reduce the risk of developing diabetes and/or clinical outcomes and complications of diabetes. In this regard, the role of the circadian system may be a potential candidate for the prevention of diabetes. We reviewed evidence from animal, clinical, and epidemiological studies linking the circadian system to various aspects of the pathophysiology and clinical outcomes of diabetes. The circadian clock governs genetic, metabolic, hormonal, and behavioral signals in anticipation of cyclic 24-hour events through interactions between a “central clock” in the suprachiasmatic nucleus and “peripheral clocks” in the whole body. Currently, circadian rhythmicity in humans can be subjectively or objectively assessed by measuring melatonin and glucocorticoid levels, core body temperature, peripheral blood, oral mucosa, hair follicles, rest-activity cycles, sleep diaries, and circadian chronotypes. In this review, we summarized various circadian misalignments, such as altered light-dark, sleep-wake, rest-activity, fasting-feeding, shift work, evening chronotype, and social jetlag, as well as mutations in clock genes that could contribute to the development of diabetes and poor glycemic status in patients with diabetes. Targeting critical components of the circadian system could deliver potential candidates for the treatment and prevention of type 2 diabetes mellitus in the future.
- Pathophysiology
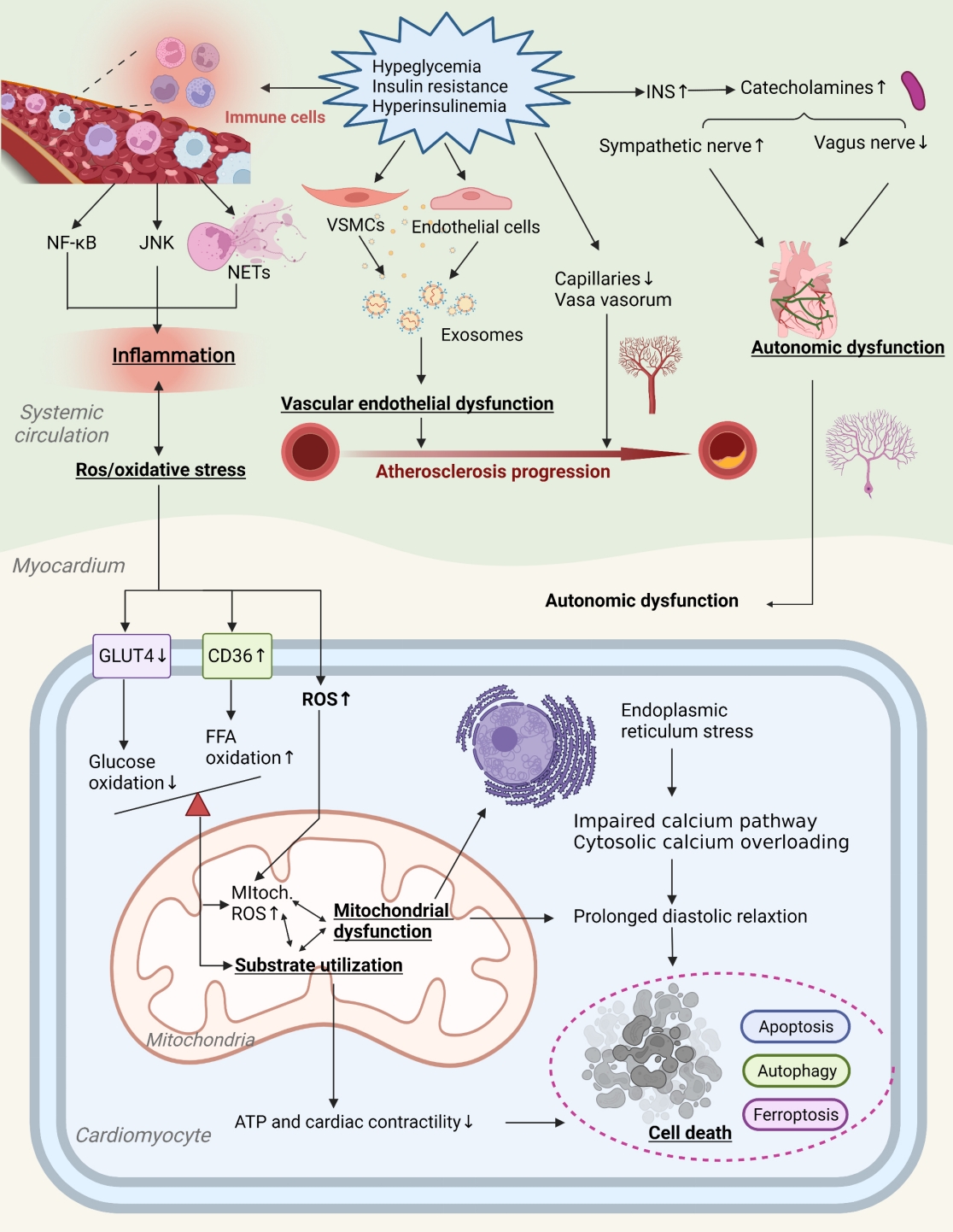
- Primordial Drivers of Diabetes Heart Disease: Comprehensive Insights into Insulin Resistance
- Yajie Fan, Zhipeng Yan, Tingting Li, Aolin Li, Xinbiao Fan, Zhongwen Qi, Junping Zhang
- Diabetes Metab J. 2024;48(1):19-36. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0110
- 2,043 View
- 178 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Insulin resistance has been regarded as a hallmark of diabetes heart disease (DHD). Numerous studies have shown that insulin resistance can affect blood circulation and myocardium, which indirectly cause cardiac hypertrophy and ventricular remodeling, participating in the pathogenesis of DHD. Meanwhile, hyperinsulinemia, hyperglycemia, and hyperlipidemia associated with insulin resistance can directly impair the metabolism and function of the heart. Targeting insulin resistance is a potential therapeutic strategy for the prevention of DHD. Currently, the role of insulin resistance in the pathogenic development of DHD is still under active research, as the pathological roles involved are complex and not yet fully understood, and the related therapeutic approaches are not well developed. In this review, we describe insulin resistance and add recent advances in the major pathological and physiological changes and underlying mechanisms by which insulin resistance leads to myocardial remodeling and dysfunction in the diabetic heart, including exosomal dysfunction, ferroptosis, and epigenetic factors. In addition, we discuss potential therapeutic approaches to improve insulin resistance and accelerate the development of cardiovascular protection drugs.
- Basic Research
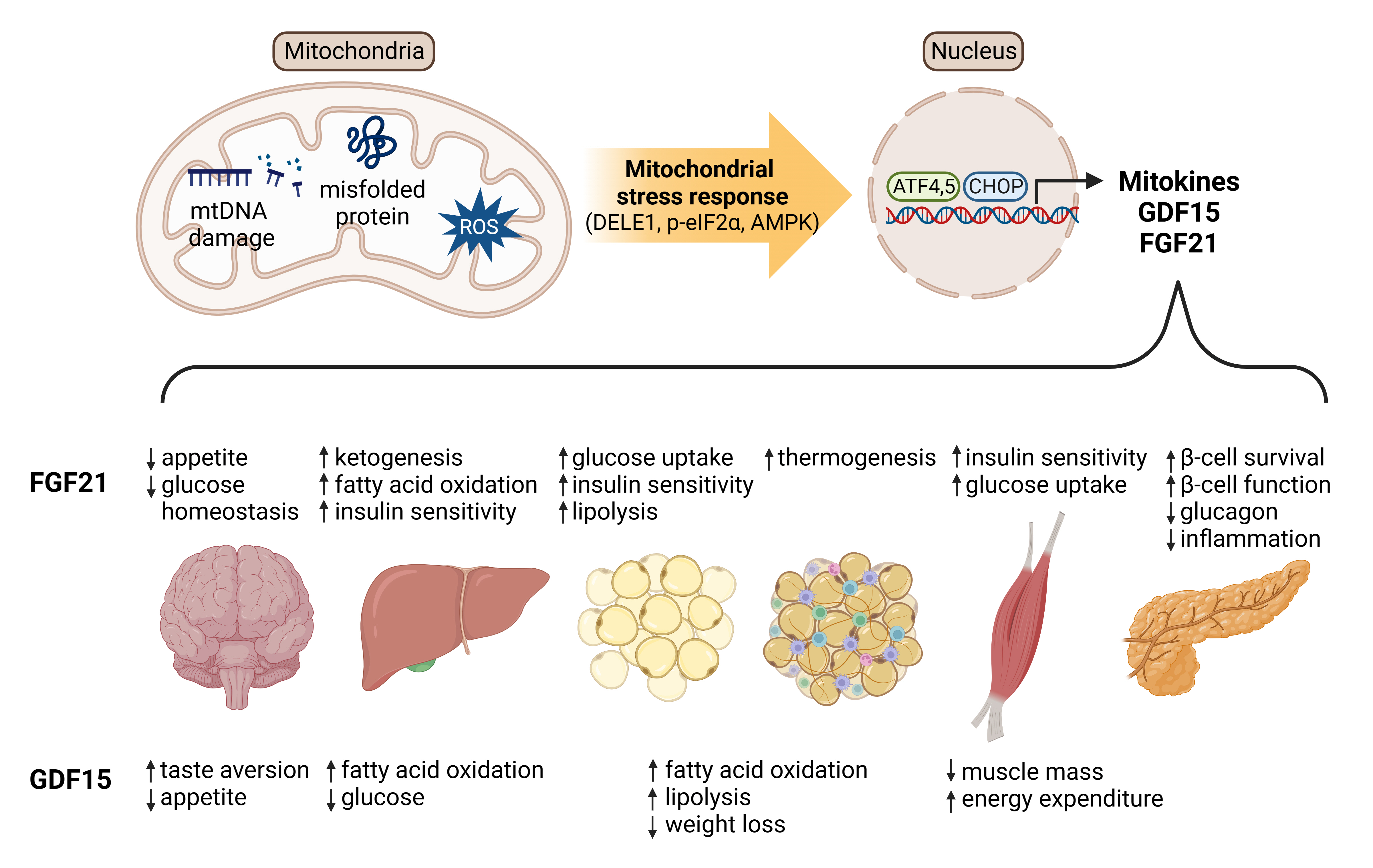
- Mitochondrial Stress and Mitokines: Therapeutic Perspectives for the Treatment of Metabolic Diseases
- Benyuan Zhang, Joon Young Chang, Min Hee Lee, Sang-Hyeon Ju, Hyon-Seung Yi, Minho Shong
- Diabetes Metab J. 2024;48(1):1-18. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0115
- 1,921 View
- 253 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Mitochondrial stress and the dysregulated mitochondrial unfolded protein response (UPRmt) are linked to various diseases, including metabolic disorders, neurodegenerative diseases, and cancer. Mitokines, signaling molecules released by mitochondrial stress response and UPRmt, are crucial mediators of inter-organ communication and influence systemic metabolic and physiological processes. In this review, we provide a comprehensive overview of mitokines, including their regulation by exercise and lifestyle interventions and their implications for various diseases. The endocrine actions of mitokines related to mitochondrial stress and adaptations are highlighted, specifically the broad functions of fibroblast growth factor 21 and growth differentiation factor 15, as well as their specific actions in regulating inter-tissue communication and metabolic homeostasis. Finally, we discuss the potential of physiological and genetic interventions to reduce the hazards associated with dysregulated mitokine signaling and preserve an equilibrium in mitochondrial stress-induced responses. This review provides valuable insights into the mechanisms underlying mitochondrial regulation of health and disease by exploring mitokine interactions and their regulation, which will facilitate the development of targeted therapies and personalized interventions to improve health outcomes and quality of life.
Response
- Glimepiride Compared to Liraglutide Increases Plasma Levels of miR-206, miR-182-5p, and miR-766-3p in Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2023;47:668-81)
- Nikolai N. Scherbak, Robert Kruse, Thomas Nyström, Johan Jendle
- Diabetes Metab J. 2023;47(6):882-883. Published online November 24, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0355
- 626 View
- 22 Download
Letter
- Glimepiride Compared to Liraglutide Increases Plasma Levels of miR-206, miR-182-5p, and miR-766-3p in Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2023;47:668-81)
- Da Young Lee
- Diabetes Metab J. 2023;47(6):879-881. Published online November 24, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0328
- 696 View
- 22 Download
Original Articles
- Complications
- The Risk of Shoulder Adhesive Capsulitis in Individuals with Prediabetes and Type 2 Diabetes Mellitus: A Longitudinal Nationwide Population-Based Study
- Jong-Ho Kim, Bong-Seoung Kim, Kyung-do Han, Hyuk-Sang Kwon
- Diabetes Metab J. 2023;47(6):869-878. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0275

- 1,465 View
- 144 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
This study aimed to investigate the association between type 2 diabetes mellitus (T2DM) and shoulder adhesive capsulitis (AC) using a large-scale, nationwide, population-based cohort in the Republic of Korea.
Methods
A total of 3,471,745 subjects aged over 20 years who underwent a National Health Insurance Service medical checkup between 2009 and 2010 were included in this study, and followed from the date of their medical checkup to the end of 2018. Subjects were classified into the following four groups based on the presence of dysglycemia and history of diabetes medication: normal, prediabetes, newly diagnosed T2DM (new-T2DM), and T2DM (claim history for antidiabetic medication). The endpoint was new-onset AC during follow-up. The incidence rates (IRs) in 1,000 person-years and hazard ratios (HRs) of AC for each group were analyzed using Cox proportional hazard regression models.
Results
The IRs of AC were 9.453 (normal), 11.912 (prediabetes), 14.933 (new-T2DM), and 24.3761 (T2DM). The adjusted HRs of AC in the prediabetes, new-T2DM, and T2DM groups were 1.084 (95% confidence interval [CI], 1.075 to 1.094), 1.312 (95% CI, 1.287 to 1.337), and 1.473 (95% CI, 1.452 to 1.494) compared to the normal group, respectively. This secular trend of the HRs of AC according to T2DM status was statistically significant (P<0.0001).
Conclusion
This large-scale, longitudinal, nationwide, population-based cohort study of 3,471,745 subjects confirmed that the risk of AC increases in prediabetic subjects and is associated with T2DM status.
- Metabolic Risk/Epidemiology
- Prediabetes Progression and Regression on Objectively- Measured Physical Function: A Prospective Cohort Study
- Shanhu Qiu, Yiming Zhu, Bo Xie, Wenji Chen, Duolao Wang, Xue Cai, Zilin Sun, Tongzhi Wu
- Diabetes Metab J. 2023;47(6):859-868. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0377

- 1,253 View
- 122 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Prediabetes leads to declines in physical function in older adults, but the impact of prediabetes progression or regression on physical function is unknown. This study assessed this longitudinal association, with physical function objectivelymeasured by grip strength, walking speed, and standing balance, based on the Health and Retirement Study enrolling United States adults aged >50 years.
Methods
Participants with prediabetes were followed-up for 4-year to ascertain prediabetes status alteration (maintained, regressed, or progressed), and another 4-year to assess their impacts on physical function. Weak grip strength was defined as <26 kg for men and <16 kg for women, slow walking speed was as <0.8 m/sec, and poor standing balance was as an uncompleted fulltandem standing testing. Logistic and linear regression analyses were performed.
Results
Of the included 1,511 participants with prediabetes, 700 maintained as prediabetes, 306 progressed to diabetes, and 505 regressed to normoglycemia over 4 years. Grip strength and walking speed were declined from baseline during the 4-year followup, regardless of prediabetes status alteration. Compared with prediabetes maintenance, prediabetes progression increased the odds of developing weak grip strength by 89% (95% confidence interval [CI], 0.04 to 2.44) and exhibited larger declines in grip strength by 0.85 kg (95% CI, –1.65 to –0.04). However, prediabetes progression was not related to impairments in walking speed or standing balance. Prediabetes regression also did not affect any measures of physical function.
Conclusion
Prediabetes progression accelerates grip strength decline in aging population, while prediabetes regression may not prevent physical function decline due to aging.
- Metabolic Risk/Epidemiology
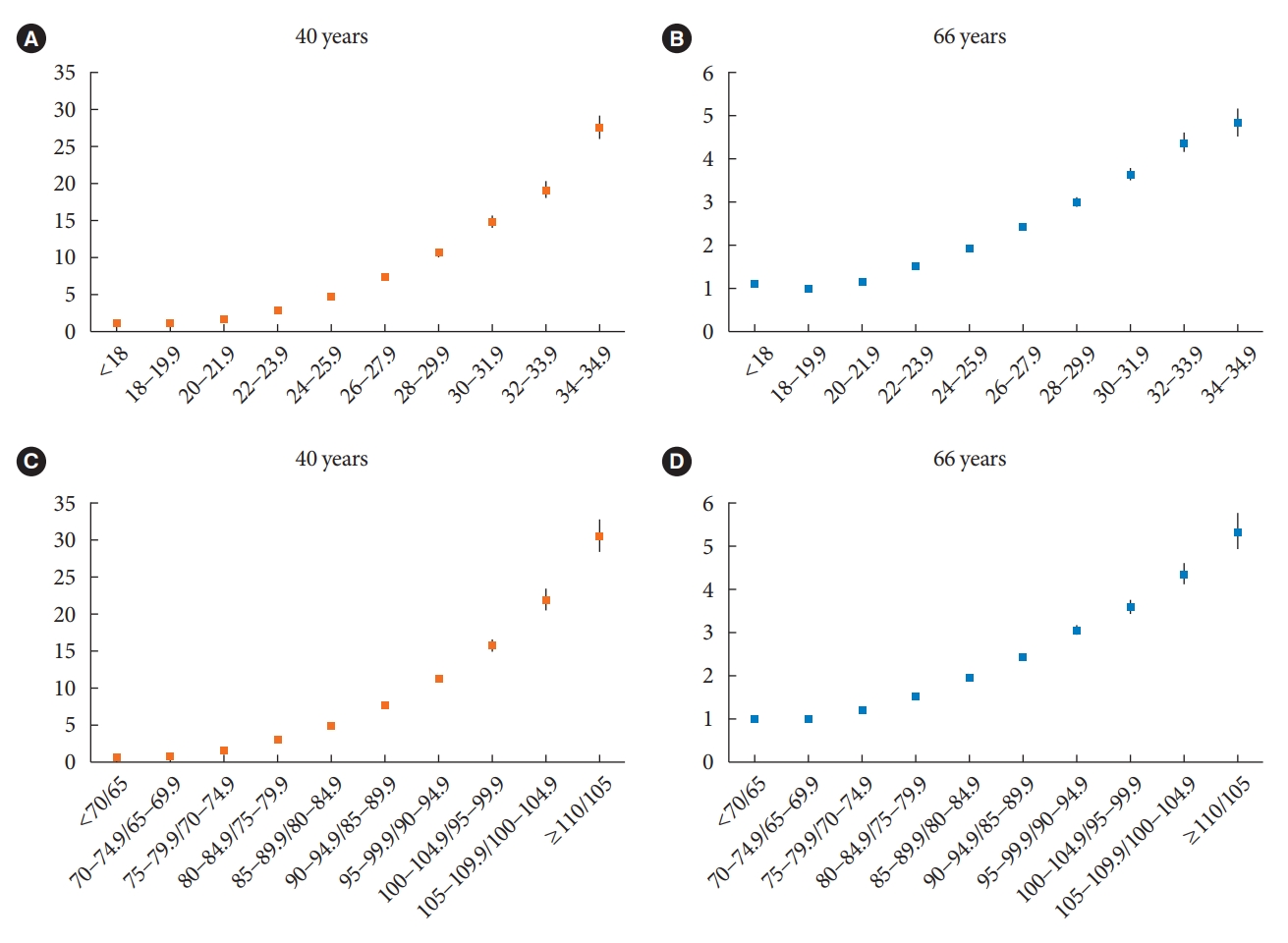
- Differential Impact of Obesity on the Risk of Diabetes Development in Two Age Groups: Analysis from the National Health Screening Program
- Tae Kyung Yoo, Kyung-Do Han, Yang-Hyun Kim, Ga Eun Nam, Sang Hyun Park, Eun-Jung Rhee, Won-Young Lee
- Diabetes Metab J. 2023;47(6):846-858. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0242
- 1,159 View
- 140 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The effect of obesity on the development of type 2 diabetes mellitus (DM) in different age groups remains unclear. We assessed the impact of obesity on the development of DM for two age groups (40-year-old, middle age; 66-year-old, older adults) in the Korean population.
Methods
We analyzed Korean National Health Insurance Service data of 4,145,321 Korean adults with 40- and 66-year-old age without DM, between 2009 and 2014. Participants were followed up until 2017 or until the diagnosis of DM. We assessed the risk of DM based on the body mass index and waist circumference of the participants. Multiple confounding factors were adjusted.
Results
The median follow-up duration was 5.6 years. The association of general and abdominal obesity with the risk of DM development was stronger in the 40-year-old group (general obesity: hazard ratio [HR], 3.566, 95% confidence interval [CI], 3.512 to 3.622; abdominal obesity: HR, 3.231; 95% CI, 3.184 to 3.278) than in the 66-year-old group (general obesity: HR, 1.739; 95% CI, 1.719 to 1.759; abdominal obesity: HR, 1.799; 95% CI, 1.778 to 1.820). In the 66-year-old group, abdominal obesity had a stronger association with the development of DM as compared to general obesity. In the 40-year-old group, general obesity had a stronger association with the risk of DM development than abdominal obesity.
Conclusion
The influence of general and abdominal obesity on the development of DM differed according to age. In older adults, abdominal obesity had a stronger association with DM development than general obesity.
- Cardiovascular Risk/Epidemiology
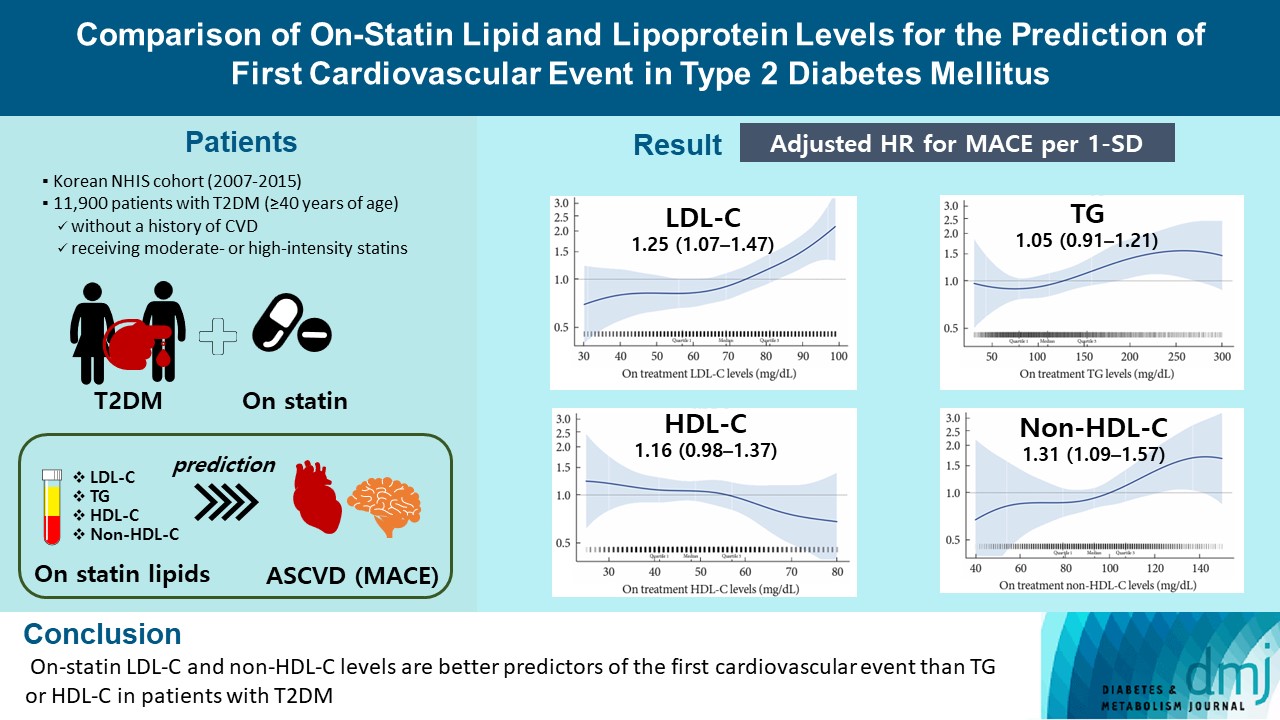
- Comparison of on-Statin Lipid and Lipoprotein Levels for the Prediction of First Cardiovascular Event in Type 2 Diabetes Mellitus
- Ji Yoon Kim, Jimi Choi, Sin Gon Kim, Nam Hoon Kim
- Diabetes Metab J. 2023;47(6):837-845. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0217
- 1,415 View
- 173 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
A substantial cardiovascular disease risk remains even after optimal statin therapy. Comparative predictiveness of major lipid and lipoprotein parameters for cardiovascular events in patients with type 2 diabetes mellitus (T2DM) who are treated with statins is not well documented.
Methods
From the Korean Nationwide Cohort, 11,900 patients with T2DM (≥40 years of age) without a history of cardiovascular disease and receiving moderate- or high-intensity statins were included. The primary outcome was the first occurrence of major adverse cardiovascular events (MACE) including ischemic heart disease, ischemic stroke, and cardiovascular death. The risk of MACE was estimated according to on-statin levels of low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), highdensity lipoprotein cholesterol (HDL-C), and non-HDL-C.
Results
MACE occurred in 712 patients during a median follow-up period of 37.9 months (interquartile range, 21.7 to 54.9). Among patients achieving LDL-C levels less than 100 mg/dL, the hazard ratios for MACE per 1-standard deviation change in ontreatment values were 1.25 (95% confidence interval [CI], 1.07 to 1.47) for LDL-C, 1.31 (95% CI, 1.09 to 1.57) for non-HDL-C, 1.05 (95% CI, 0.91 to 1.21) for TG, and 1.16 (95% CI, 0.98 to 1.37) for HDL-C, after adjusting for potential confounders and lipid parameters mutually. The predictive ability of on-statin LDL-C and non-HDL-C for MACE was prominent in patients at high cardiovascular risk or those with LDL-C ≥70 mg/dL.
Conclusion
On-statin LDL-C and non-HDL-C levels are better predictors of the first cardiovascular event than TG or HDL-C in patients with T2DM.
- Technology/Device
- Clinical and Lifestyle Determinants of Continuous Glucose Monitoring Metrics in Insulin-Treated Patients with Type 2 Diabetes Mellitus
- Da Young Lee, Namho Kim, Inha Jung, So Young Park, Ji Hee Yu, Ji A Seo, Jihee Kim, Kyeong Jin Kim, Nam Hoon Kim, Hye Jin Yoo, Sin Gon Kim, Kyung Mook Choi, Sei Hyun Baik, Sung-Min Park, Nan Hee Kim
- Diabetes Metab J. 2023;47(6):826-836. Published online August 24, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0273

- 1,738 View
- 190 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There was limited evidence to evaluate the association between lifestyle habits and continuous glucose monitoring (CGM) metrics. Thus, we aimed to depict the behavioral and metabolic determinants of CGM metrics in insulin-treated patients with type 2 diabetes mellitus (T2DM).
Methods
This is a prospective observational study. We analyzed data from 122 insulin-treated patients with T2DM. Participants wore Dexcom G6 and Fitbit, and diet information was identified for 10 days. Multivariate-adjusted logistic regression analysis was performed for the simultaneous achievement of CGM-based targets, defined by the percentage of time in terms of hyper, hypoglycemia and glycemic variability (GV). Intake of macronutrients and fiber, step counts, sleep, postprandial C-peptide-to-glucose ratio (PCGR), information about glucose lowering medications and metabolic factors were added to the analyses. Additionally, we evaluated the impact of the distribution of energy and macronutrient during a day, and snack consumption on CGM metrics.
Results
Logistic regression analysis revealed that female, participants with high PCGR, low glycosylated hemoglobin (HbA1c) and daytime step count had a higher probability of achieving all targets based on CGM (odds ratios [95% confidence intervals] which were 0.24 [0.09 to 0.65], 1.34 [1.03 to 1.25], 0.95 [0.9 to 0.99], and 1.15 [1.03 to 1.29], respectively). And participants who ate snacks showed a shorter period of hyperglycemia and less GV compared to those without.
Conclusion
We confirmed that residual insulin secretion, daytime step count, HbA1c, and women were the most relevant determinants of adequate glycemic control in insulin-treated patients with T2DM. In addition, individuals with snack consumption were exposed to lower times of hyperglycemia and GV. -
Citations
Citations to this article as recorded by- Explanatory variables of objectively measured 24-h movement behaviors in people with prediabetes and type 2 diabetes: A systematic review
Lotte Bogaert, Iris Willems, Patrick Calders, Eveline Dirinck, Manon Kinaupenne, Marga Decraene, Bruno Lapauw, Boyd Strumane, Margot Van Daele, Vera Verbestel, Marieke De Craemer
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2024; 18(4): 102995. CrossRef
- Explanatory variables of objectively measured 24-h movement behaviors in people with prediabetes and type 2 diabetes: A systematic review
- Drug Regimen
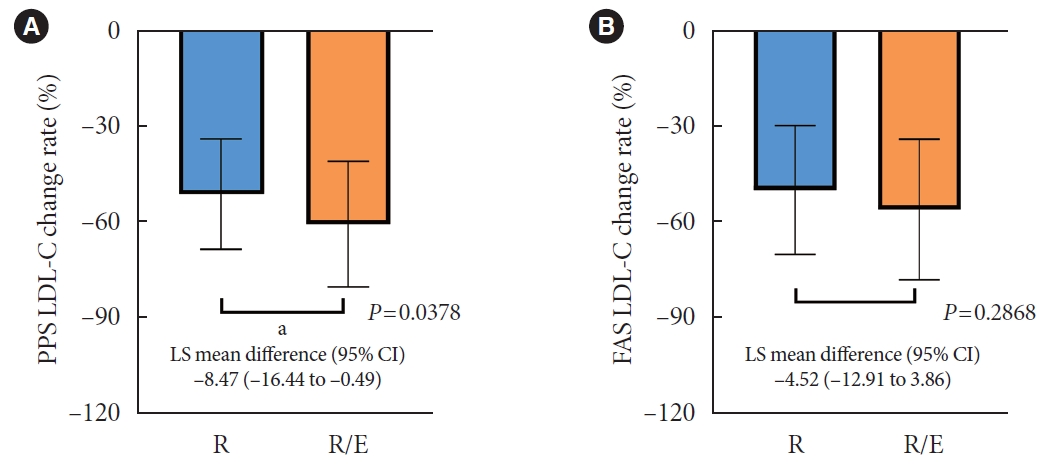
- The Efficacy and Safety of Moderate-Intensity Rosuvastatin with Ezetimibe versus High-Intensity Rosuvastatin in High Atherosclerotic Cardiovascular Disease Risk Patients with Type 2 Diabetes Mellitus: A Randomized, Multicenter, Open, Parallel, Phase 4 Study
- Jun Sung Moon, Il Rae Park, Sang Soo Kim, Hye Soon Kim, Nam Hoon Kim, Sin Gon Kim, Seung Hyun Ko, Ji Hyun Lee, Inkyu Lee, Bo Kyeong Lee, Kyu Chang Won
- Diabetes Metab J. 2023;47(6):818-825. Published online November 24, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0171
- 2,270 View
- 239 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
To investigate the efficacy and safety of moderate-intensity rosuvastatin/ezetimibe combination compared to highintensity rosuvastatin in high atherosclerotic cardiovascular disease (ASCVD) risk patients with type 2 diabetes mellitus (T2DM).
Methods
This study was a randomized, multicenter, open, parallel phase 4 study, and enrolled T2DM subjects with an estimated 10-year ASCVD risk ≥7.5%. The primary endpoint was the low-density lipoprotein cholesterol (LDL-C) change rate after 24-week rosuvastatin 10 mg/ezetimibe 10 mg treatment was non-inferior to that of rosuvastatin 20 mg. The achievement proportion of 10-year ASCVD risk <7.5% or comprehensive lipid target (LDL-C <70 mg/dL, non-high-density lipoprotein cholesterol <100 mg/dL, and apolipoprotein B <80 mg/dL) without discontinuation, and several metabolic parameters were explored as secondary endpoints.
Results
A hundred and six participants were assigned to each group. Both groups showed significant reduction in % change of LDL-C from baseline at week 24 (–63.90±6.89 vs. –55.44±6.85, combination vs. monotherapy, p=0.0378; respectively), but the combination treatment was superior to high-intensity monotherapy in LDL-C change (%) from baseline (least square [LS] mean difference, –8.47; 95% confidence interval, –16.44 to –0.49; p=0.0378). The combination treatment showed a higher proportion of achieved comprehensive lipid targets rather than monotherapy (85.36% vs. 62.22% in monotherapy, p=0.015). The ezetimibe combination significantly improved homeostasis model assessment of β-cell function even without A1c changes (LS mean difference, 17.13; p=0.0185).
Conclusion
In high ASCVD risk patients with T2DM, the combination of moderate-intensity rosuvastatin and ezetimibe was not only non-inferior but also superior to improving dyslipidemia with additional benefits compared to high-intensity rosuvastatin monotherapy.
- Drug Regimen
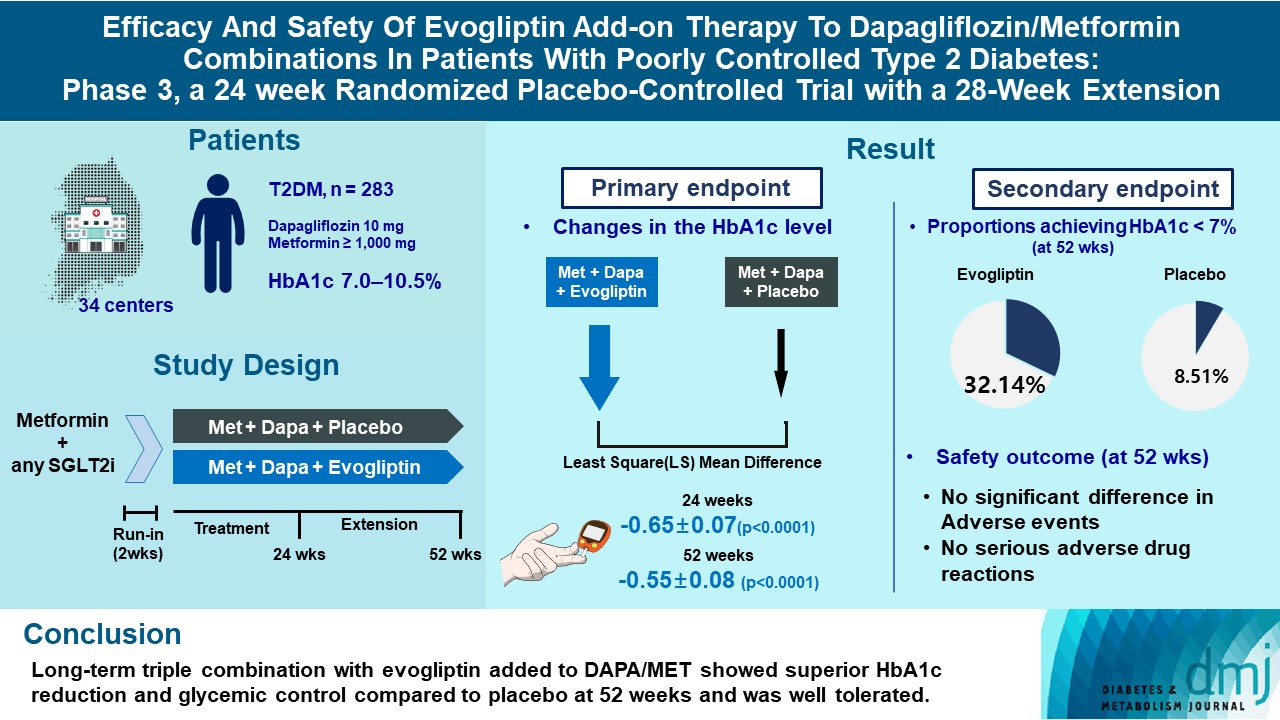
- Efficacy and Safety of Evogliptin Add-on Therapy to Dapagliflozin/Metformin Combinations in Patients with Poorly Controlled Type 2 Diabetes Mellitus: A 24-Week Multicenter Randomized Placebo-Controlled Parallel-Design Phase-3 Trial with a 28-Week Extension
- Jun Sung Moon, Il Rae Park, Hae Jin Kim, Choon Hee Chung, Kyu Chang Won, Kyung Ah Han, Cheol-Young Park, Jong Chul Won, Dong Jun Kim, Gwan Pyo Koh, Eun Sook Kim, Jae Myung Yu, Eun-Gyoung Hong, Chang Beom Lee, Kun-Ho Yoon
- Diabetes Metab J. 2023;47(6):808-817. Published online September 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0387
- 2,514 View
- 277 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study investigates the long-term efficacy and safety of evogliptin add-on therapy in patients with inadequately controlled type 2 diabetes mellitus (T2DM) previously received dapagliflozin and metformin (DAPA/MET) combination.
Methods
In this multicenter randomized placebo-controlled phase 3 trial, patients with glycosylated hemoglobin (HbA1c) levels 7.0% to 10.5% (n=283) previously used DAPA 10 mg plus MET (≥1,000 mg) were randomly assigned to the evogliptin 5 mg once daily or placebo group (1:1). The primary endpoint was the difference in the HbA1c level from baseline at week 24, and exploratory endpoints included the efficacy and safety of evogliptin over 52 weeks (trial registration: ClinicalTrials.gov NCT04170998).
Results
Evogliptin add-on to DAPA/MET therapy was superior in HbA1c reduction compared to placebo at weeks 24 and 52 (least square [LS] mean difference, –0.65% and –0.55%; 95% confidence interval [CI], –0.79 to –0.51 and –0.71 to –0.39; P<0.0001). The proportion of patients achieving HbA1c <7% was higher in the triple combination group at week 52 (32.14% vs. 8.51% in placebo; odds ratio, 5.62; P<0.0001). Evogliptin significantly reduced the fasting glucose levels and mean daily glucose levels with improvement in homeostatic model assessment of β-cell function (LS mean difference, 9.04; 95% CI, 1.86 to 16.21; P=0.0138). Adverse events were similar between the groups, and no serious adverse drug reactions were reported in the evogliptin group.
Conclusion
Long-term triple combination with evogliptin added to DAPA/MET showed superior HbA1c reduction and glycemic control compared to placebo at 52 weeks and was well tolerated.
- Drug Regimen
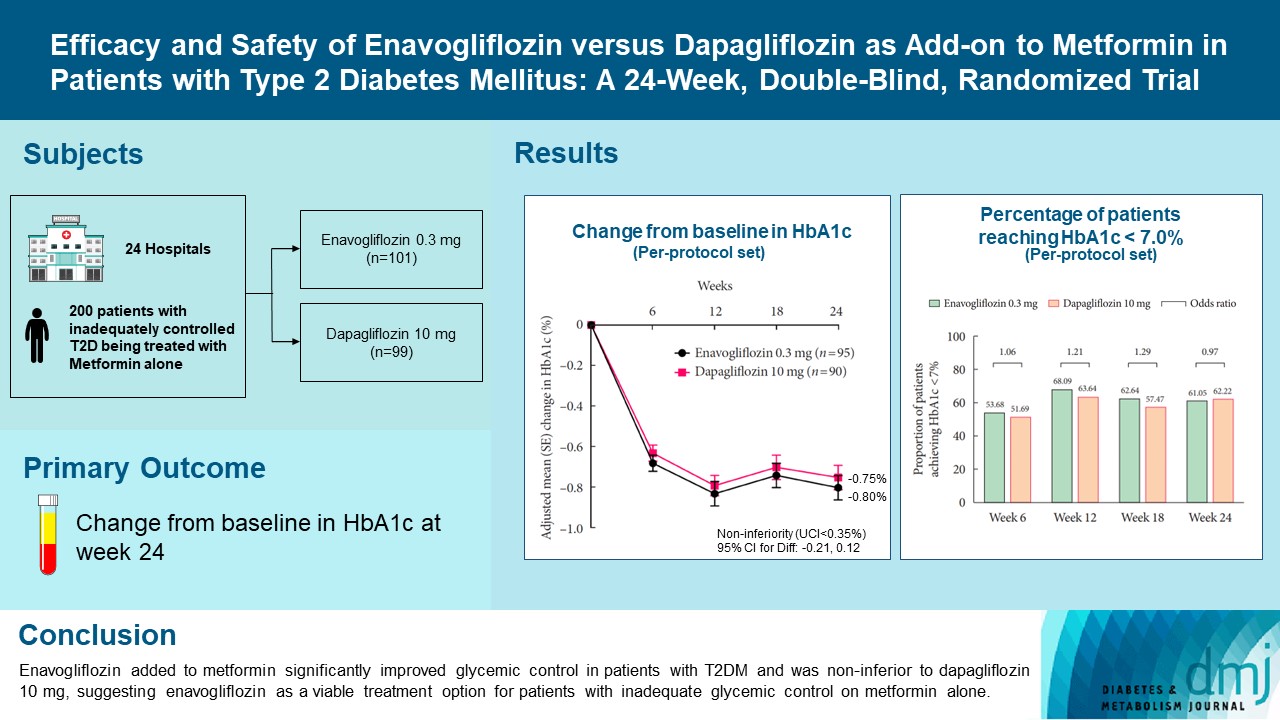
- Efficacy and Safety of Enavogliflozin versus Dapagliflozin as Add-on to Metformin in Patients with Type 2 Diabetes Mellitus: A 24-Week, Double-Blind, Randomized Trial
- Kyung Ah Han, Yong Hyun Kim, Doo Man Kim, Byung Wan Lee, Suk Chon, Tae Seo Sohn, In Kyung Jeong, Eun-Gyoung Hong, Jang Won Son, Jae Jin Nah, Hwa Rang Song, Seong In Cho, Seung-Ah Cho, Kun Ho Yoon
- Diabetes Metab J. 2023;47(6):796-807. Published online February 9, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0315
- 39,932 View
- 563 Download
- 3 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Enavogliflozin is a novel sodium-glucose cotransporter-2 inhibitor currently under clinical development. This study evaluated the efficacy and safety of enavogliflozin as an add-on to metformin in Korean patients with type 2 diabetes mellitus (T2DM) against dapagliflozin.
Methods
In this multicenter, double-blind, randomized, phase 3 study, 200 patients were randomized to receive enavogliflozin 0.3 mg/day (n=101) or dapagliflozin 10 mg/day (n=99) in addition to ongoing metformin therapy for 24 weeks. The primary objective of the study was to prove the non-inferiority of enavogliflozin to dapagliflozin in glycosylated hemoglobin (HbA1c) change at week 24 (non-inferiority margin of 0.35%) (Clinical trial registration number: NCT04634500).
Results
Adjusted mean change of HbA1c at week 24 was –0.80% with enavogliflozin and –0.75% with dapagliflozin (difference, –0.04%; 95% confidence interval, –0.21% to 0.12%). Percentages of patients achieving HbA1c <7.0% were 61% and 62%, respectively. Adjusted mean change of fasting plasma glucose at week 24 was –32.53 and –29.14 mg/dL. An increase in urine glucose-creatinine ratio (60.48 vs. 44.94, P<0.0001) and decrease in homeostasis model assessment of insulin resistance (–1.85 vs. –1.31, P=0.0041) were significantly greater with enavogliflozin than dapagliflozin at week 24. Beneficial effects of enavogliflozin on body weight (–3.77 kg vs. –3.58 kg) and blood pressure (systolic/diastolic, –5.93/–5.41 mm Hg vs. –6.57/–4.26 mm Hg) were comparable with those of dapagliflozin, and both drugs were safe and well-tolerated.
Conclusion
Enavogliflozin added to metformin significantly improved glycemic control in patients with T2DM and was non-inferior to dapagliflozin 10 mg, suggesting enavogliflozin as a viable treatment option for patients with inadequate glycemic control on metformin alone. -
Citations
Citations to this article as recorded by- Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
Young Sang Lyu, Sangmo Hong, Si Eun Lee, Bo Young Cho, Cheol-Young Park
Cardiovascular Diabetology.2024;[Epub] CrossRef - A 52‐week efficacy and safety study of enavogliflozin versus dapagliflozin as an add‐on to metformin in patients with type 2 diabetes mellitus: ENHANCE‐M extension study
Tae Seo Sohn, Kyung‐Ah Han, Yonghyun Kim, Byung‐Wan Lee, Suk Chon, In‐Kyung Jeong, Eun‐Gyoung Hong, Jang Won Son, JaeJin Na, Jae Min Cho, Seong In Cho, Wan Huh, Kun‐Ho Yoon
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Role of novel sodium glucose co-transporter-2 inhibitor enavogliflozin in type-2 diabetes: A systematic review and meta-analysis
Deep Dutta, B.G. Harish, Beatrice Anne, Lakshmi Nagendra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(8): 102816. CrossRef - Characteristics of the Latest Therapeutic Agent for Diabetes
Nuri Yun
The Journal of Korean Diabetes.2023; 24(3): 148. CrossRef - Prospects of using sodium-glucose co-transporter-2 (SGLT-2) inhibitors in patients with metabolic-associated fatty liver disease (MAFLD)
Iryna Kostitska, Nadia Protas, Liliia Petrovska
Diabetes Obesity Metabolic Syndrome.2023; (5): 8. CrossRef - Navigating the Future of Diabetes Treatment with New Drugs: Focusing on the Possibilities and Prospects of Enavogliflozin
Sang Youl Rhee
Diabetes & Metabolism Journal.2023; 47(6): 769. CrossRef
- Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
- Basic Research
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition
- Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
- Diabetes Metab J. 2023;47(6):784-795. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0261
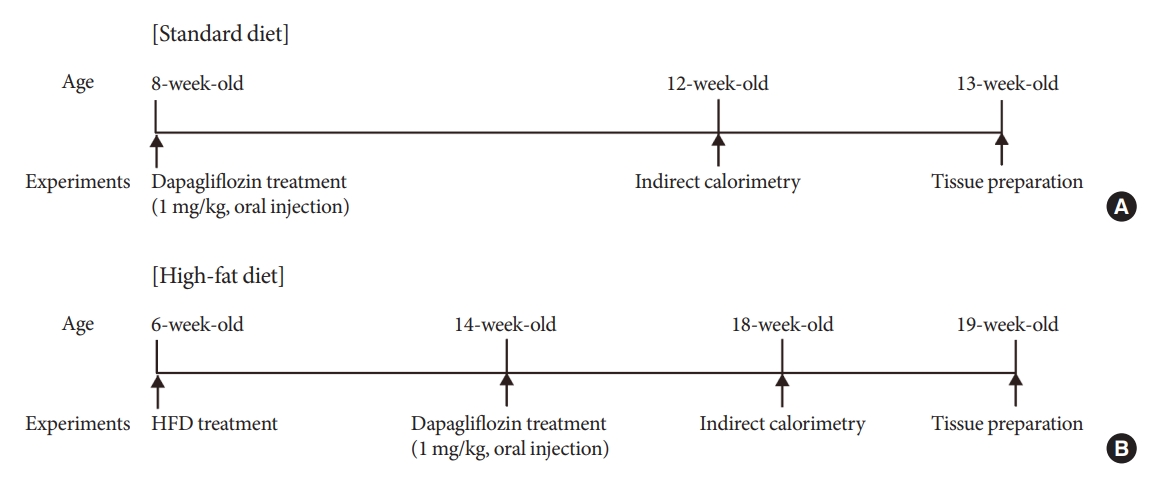
- 1,361 View
- 149 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Sodium-glucose cotransporter 2 (SGLT-2) inhibitors are currently used to treat patients with diabetes. Previous studies have demonstrated that treatment with SGLT-2 inhibitors is accompanied by altered metabolic phenotypes. However, it has not been investigated whether the hypothalamic circuit participates in the development of the compensatory metabolic phenotypes triggered by the treatment with SGLT-2 inhibitors.
Methods
Mice were fed a standard diet or high-fat diet and treated with dapagliflozin, an SGLT-2 inhibitor. Food intake and energy expenditure were observed using indirect calorimetry system. The activity of hypothalamic neurons in response to dapagliflozin treatment was evaluated by immunohistochemistry with c-Fos antibody. Quantitative real-time polymerase chain reaction was performed to determine gene expression patterns in the hypothalamus of dapagliflozin-treated mice.
Results
Dapagliflozin-treated mice displayed enhanced food intake and reduced energy expenditure. Altered neuronal activities were observed in multiple hypothalamic nuclei in association with appetite regulation. Additionally, we found elevated immunosignals of agouti-related peptide neurons in the paraventricular nucleus of the hypothalamus.
Conclusion
This study suggests the functional involvement of the hypothalamus in the development of the compensatory metabolic phenotypes induced by SGLT-2 inhibitor treatment. -
Citations
Citations to this article as recorded by- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Jae Hyun Bae
Diabetes & Metabolism Journal.2024; 48(1): 157. CrossRef - Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
Diabetes & Metabolism Journal.2024; 48(1): 159. CrossRef
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
- Basic Research
- Effects Of Exercise Training And Chlorogenic Acid Supplementation On Hepatic Lipid Metabolism In Prediabetes Mice
- Samaneh Shirkhani, Sayyed Mohammad Marandi, Mohammad Hossein Nasr-Esfahani, Seung Kyum Kim
- Diabetes Metab J. 2023;47(6):771-783. Published online September 8, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0265

- 2,207 View
- 166 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Since prediabetes is a risk factor for metabolic syndromes, it is important to promote a healthy lifestyle to prevent prediabetes. This study aimed to determine the effects of green coffee (GC), chlorogenic acid (CGA) intake, and exercise training (EX) on hepatic lipid metabolism in prediabetes male C57BL/6 mice.
Methods
Forty-nine mice were randomly divided into two groups feeding with a normal diet (n=7) or a high-fat diet (HFD, n=42) for 12 weeks. Then, HFD mice were further divided into six groups (n=7/group): control (pre-D), GC, CGA, EX, GC+EX, and CGA+EX. After additional 10 weeks under the same diet, plasma, and liver samples were obtained.
Results
HFD-induced prediabetes conditions with increases in body weight, glucose, insulin, insulin resistance, and lipid profiles were alleviated in all treatment groups. Acsl3, a candidate gene identified through an in silico approach, was lowered in the pre-D group, while treatments partly restored it. HFD induced adverse alterations of de novo lipogenesis- and β oxidation-associated molecules in the liver. However, GC and CGA supplementation and EX reversed or ameliorated these changes. In most cases, GC or CGA supplementation combined with EX has no synergistic effect and the GC group had similar results to the CGA group.
Conclusion
These findings suggest that regular exercise is an effective non-therapeutic approach for prediabetes, and CGA supplementation could be an alternative to partially mimic the beneficial effects of exercise on prediabetes. -
Citations
Citations to this article as recorded by- Research progress on the pharmacological activity and mechanism of chlorogenic acid in alleviating acute kidney injury in sepsis patients
Perioperative Precision Medicine.2023;[Epub] CrossRef
- Research progress on the pharmacological activity and mechanism of chlorogenic acid in alleviating acute kidney injury in sepsis patients

 KDA
KDA
 First
First Prev
Prev





