Exenatide versus Insulin Lispro Added to Basal Insulin in a Subgroup of Korean Patients with Type 2 Diabetes Mellitus
Article information
Abstract
Background
The prevalence of type 2 diabetes mellitus (T2DM) and obesity is increasing in Korea. Clinical studies in patients with T2DM have shown that combining the glucagon-like peptide-1 receptor agonist exenatide twice daily with basal insulin is an effective glucose-lowering strategy. However, these studies were predominantly conducted in non-Asian populations.
Methods
We conducted a subgroup analysis of data from a multinational, 30-week, randomized, open-label trial to compare the effects of exenatide twice daily (n=10) or three times daily mealtime insulin lispro (n=13) among Korean patients with T2DM inadequately controlled (glycosylated hemoglobin [HbA1c] >7.0%) on metformin plus optimized insulin glargine.
Results
Exenatide twice daily and insulin lispro both reduced HbA1c (mean −1.5% and −1.0%, respectively; P<0.01 vs. baseline). Fasting glucose and weight numerically decreased with exenatide twice daily (−0.7 mmol/L and −0.7 kg, respectively) and numerically increased with insulin lispro (0.9 mmol/L and 1.0 kg, respectively). Minor hypoglycemia occurred in four patients receiving exenatide twice daily and three patients receiving insulin lispro. Gastrointestinal adverse events were the most common with exenatide twice daily treatment.
Conclusion
This analysis found treatment with exenatide twice daily improved glycemic control without weight gain in Korean patients with T2DM unable to achieve glycemic control on metformin plus basal insulin.
INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) is increasing in Asian countries, with some reporting comparable or higher rates of diabetes than Western countries [1]. In Korea, diabetes prevalence increased from 7.2% in 1993 to 9.9% over 2007 to 2009 [2]. The World Health Organization notes that increased T2DM risk is seen at a lower body mass index (BMI) for Asian compared with European populations [3]; however, the prevalence of obesity (BMI ≥25 kg/m2) is also increasing in Korea, and 40% to 50% of Korean people with diabetes are now obese [45].
Although postprandial glucose (PPG) and fasting glucose (FG) levels both play an important role in the development of hyperglycemia in patients with T2DM, elevated PPG appears to be a greater contributor to hyperglycemia than FG among Asian patients [67]. For patients unable to achieve glycemic targets with oral glucose-lowering agents or a single injectable, international guidelines recommend combination injectable therapy [8]. One such option includes the combination of basal insulin and a glucagon-like peptide-1 receptor agonist (GLP-1RA). GLP-1RAs improve glycemic control through increased glucose-dependent insulin secretion, glucose-dependent suppression of glucagon, delayed gastric emptying, and increased satiety [9]. The short-acting GLP-1RA exenatide twice daily is dosed with breakfast and dinner, specifically reducing the PPG excursions that occur with these meals [10]. The glucose-lowering effectiveness of combining exenatide twice daily with basal insulin has previously been demonstrated without the weight gain and hypoglycemia associated with complex basal-bolus insulin regimens [1112]. However, treatment responses to glucose-lowering medications may vary across populations with different disease phenotypes and/or regional practices, and the addition of exenatide twice daily to basal insulin has been primarily studied in non-Asian populations [11,12]. Furthermore, to our knowledge, there is no published evidence of the efficacy and safety of exenatide twice daily specifically in Korean patients receiving basal insulin.
In the Basal Insulin Glargine plus Exenatide twice daily versus Basal Insulin Glargine plus Bolus Insulin Lispro (4B) study, addition of exenatide twice daily or mealtime insulin lispro to treatment among patients with T2DM and insufficient glycemic control on titrated insulin glargine resulted in comparable reductions in glycosylated hemoglobin (HbA1c) [12]. However, compared with insulin lispro, exenatide twice daily treatment resulted in weight loss rather than weight gain and fewer nonnocturnal hypoglycemia episodes [12]. Here, we report the results of a subgroup analysis examining the efficacy and tolerability of exenatide twice daily among patients enrolled in the 4B study at centers in South Korea.
METHODS
Study design and patients
The protocol was approved by Ethics and Regulatory Committees, the Institutional Review Boards, and was completed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients provided written informed consent [12].
The methods of the randomized, controlled, open-label, noninferiority 4B study (NCT00960661) were reported previously [12]. Briefly, eligible patients were males and females aged ≥18 years with T2DM treated with insulin glargine and metformin, with an HbA1c of 7.0% to 10.0%. Eligible South Korean patients had a BMI of 23.0 kg/m2 (vs. 25.0 kg/m2 for all others) to 45.0 kg/m2. The study comprised a 12-week basal insulin optimization phase, during which insulin glargine was titrated to an FG of ≤5.6 mmol/L without hypoglycemia. Patients who were unable to reach HbA1c ≤7.0% were randomized to receive exenatide (5 µg twice daily for 4 weeks, then 10 µg twice daily) before the two largest meals with ≥6 hours between dosing or three times daily mealtime insulin lispro (titrated to achieve preprandial blood glucose <6.1 mmol/L without hypoglycemia), both added to insulin glargine and metformin, for the 30-week intervention phase. Insulin glargine dose was reduced by ≥10% for patients with an HbA1c of ≤8.0% receiving exenatide twice daily or by one-half or one-third for patients receiving insulin lispro. Thereafter, insulin glargine was titrated as in the basal insulin optimization phase.
Study outcome measures
The primary outcome was the change in HbA1c from randomization to 30 weeks [12]. Key secondary outcomes included achievement of HbA1c targets (≤6.5%, ≤7.0%), total insulin dose, seven-point self-monitored blood glucose profiles, and changes in FG and weight. Safety outcomes included hypoglycemia and adverse events (AEs).
Statistical analysis
Patient characteristics and efficacy outcomes were reported for the per-protocol population, defined as all randomized patients completing the study who met the inclusion, exclusion, and discontinuation criteria [12]. Safety analyses were reported for the as-treated population, defined as all randomized patients who received at least one dose of study medication, according to treatment received. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). Categorical variables were reported as frequency (percentage) of patients, and continuous variables as arithmetic mean±standard deviation (SD), for each treatment group. Endpoint data were generated using the last observation carried forward method, and P values were derived from comparisons to baseline using paired t-tests.
RESULTS
Patient disposition and demographics
Thirty Korean patients were randomized to receive exenatide twice daily (n=15) or insulin lispro (n=15). In the per-protocol population, 10 Korean patients received exenatide twice daily and 13 received three times daily mealtime insulin lispro. Numerically, baseline characteristics were well balanced between the two treatment groups; however, statistical comparisons were not conducted (Table 1). Reasons for discontinuation with exenatide twice daily were AE (n=1), protocol violation (n=3), and sponsor decision (n=1). Two patients receiving insulin lispro discontinued due to patient decision.
Efficacy
Significant (P<0.05) reductions from baseline in HbA1c were observed for both groups from week 2 onwards (Fig. 1A); at endpoint, the mean±SD change from baseline was −1.5%± 1.2% with exenatide twice daily (P=0.004) and −1.0%±1.0% with insulin lispro (P=0.002). HbA1c goals of ≤7.0% and ≤6.5% were achieved by 60% and 50% of exenatide twice daily-treated patients, respectively, and by 46% and 23% of insulin lispro-treated patients, respectively. Insulin glargine dose was reduced from baseline throughout the study in both treatment groups but was consistently higher in the exenatide twice daily group (Fig. 1B). Mean±SD daily insulin glargine doses were 42.3±10.1 units/day with exenatide twice daily and 42.0±19.0 units/day with insulin lispro at baseline, and 39.2±13.9 units/day with exenatide twice daily and 35.5±16.1 units/day with insulin lispro at endpoint. Compared with baseline, FG numerically decreased with exenatide twice daily and numerically increased with insulin lispro, with significant increases at weeks 6 and 18 (Fig. 1C). The mean±SD change in FG from baseline was −0.7±1.6 mmol/L with exenatide twice daily (P=0.23) and 0.9±1.9 mmol/L with insulin lispro (P=0.11). PPG tended to decrease from baseline in both treatment groups (Fig. 1D). Relative to baseline, weight numerically decreased in exenatide twice daily-treated patients, with significant reductions at weeks 6 and 8 (P<0.05) (Fig. 1E). At endpoint, there was a −0.7±3.3 kg change in weight from baseline with exenatide twice daily (P=0.50) and a 1.0±2.7 kg change in weight with insulin lispro (P=0.21).
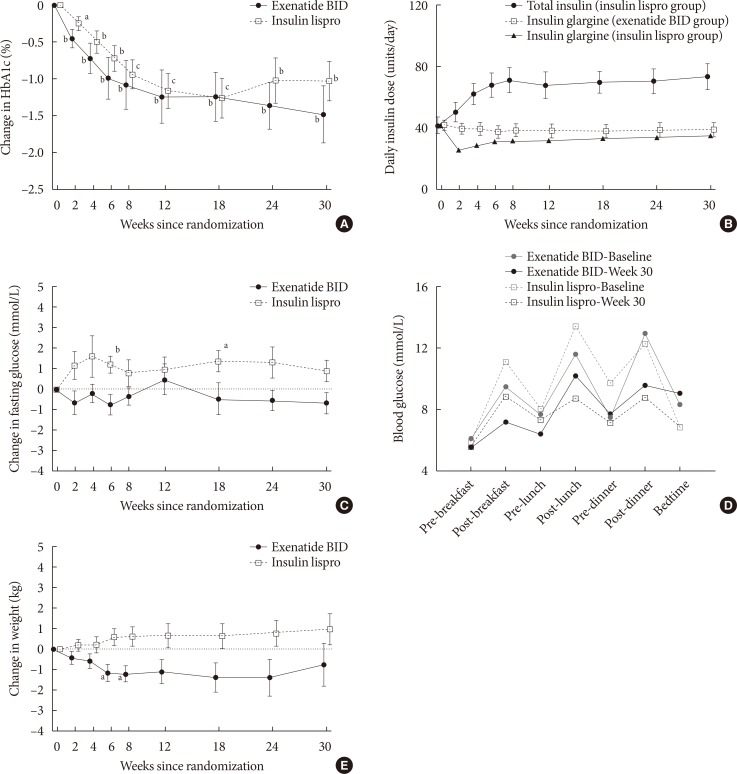
Primary and secondary outcomes over 30 weeks for the per-protocol population: (A) change in glycosylated hemoglobin (HbA1c) values (primary outcome measure); (B) insulin glargine dose in the exenatide twice daily (BID) treatment group (open squares) and the insulin lispro treatment group (closed triangles), and total insulin dose in the insulin lispro group (closed circles); (C) change in fasting glucose; (D) self-monitored blood glucose values at randomization (gray lines) and 30 weeks (black lines); and (E) change in weight. Values are presented as mean±standard error. aP<0.05, bP≤0.01, cP<0.001.
Safety and tolerability
The most common AEs were gastrointestinal. In the exenatide twice daily and insulin lispro groups, this included nausea (five patients [33%] and 0, respectively), constipation (0 and two [13%]), and vomiting (two [13%] and 0). Serious AEs were reported by one patient in each group (exenatide twice daily: fall and lower-limb fracture leading to study discontinuation; insulin lispro: acute pyelonephritis and nephrolithiasis). The investigators judged both serious AEs as not related to study medication.
No major hypoglycemia events occurred in either group. Four patients receiving exenatide twice daily and three patients receiving insulin lispro experienced minor hypoglycemia events (12 events vs. eight events, respectively). More nocturnal than daytime hypoglycemia events occurred with exenatide twice daily (four patients vs. two patients [10 events vs. two events]) and more daytime than nocturnal hypoglycemia occurred with insulin lispro (three patients vs. one patient [seven events vs. one event]).
DISCUSSION
In this analysis of the subpopulation of Korean patients participating in the 4B study, we found that exenatide twice daily administered before the two largest meals and prandial insulin lispro both effectively reduced HbA1c in patients with poor glycemic control despite titrated basal insulin. FG and weight both numerically decreased in patients treated with exenatide twice daily and numerically increased in patients receiving insulin lispro. Minor daytime and minor nocturnal hypoglycemic events occurred most frequently in the insulin lispro and exenatide twice daily groups, respectively. Gastrointestinal AEs were most frequently reported: nausea and vomiting were most common with exenatide twice daily, and constipation occurred most often with insulin lispro.
Collectively, changes in indices of glycemic control in the Korean subpopulation were consistent with the findings of the overall 4B study, which predominantly enrolled white patients (88%) [12]. The primary study reported a reduction in HbA1c of −1.13% with exenatide twice daily and −1.10% with insulin lispro. Despite similar reductions in HbA1c, there was a significant between group difference regarding the change in FG (−0.5 mmol/L for exenatide twice daily vs. 0.2 mmol/L for insulin lispro). Similarly, among Korean patients, HbA1c and FG decreased with exenatide twice daily, HbA1c decreased with insulin lispro, and FG increased with insulin lispro. Additionally, among the primary study population, treatment with exenatide twice daily resulted in weight loss (−2.5 kg), whereas treatment with insulin lispro resulted in weight gain (2.1 kg) [12]. Likewise, patients in the Korean subgroup receiving exenatide twice daily lost weight and patients receiving insulin lispro gained weight. For the Korean subgroup, this small decrease in weight could be important over time, especially as the prevalence of severe obesity has increased among Korean patients with diabetes [4].
In the primary study, nocturnal hypoglycemia occurred at a similar frequency among exenatide twice daily- and insulin lispro-treated patients [12]. Nocturnal hypoglycemia was likely attributable to insulin glargine, as both exenatide twice daily and insulin lispro have short half-lives and are administered during the day, whereas insulin glargine is administered at bedtime [12]. In the Korean subpopulation randomized to exenatide twice daily, the decrease in FG, relatively small insulin glargine dose reduction at randomization, relatively high insulin glargine dose at endpoint, and the relatively high frequency of nocturnal hypoglycemia collectively suggest that physicians may have aggressively titrated insulin glargine when given with exenatide twice daily.
The findings of the current study are also consistent with the effects of other studies of GLP-1RAs. A study of exenatide twice daily added to insulin glargine versus insulin glargine alone also found add-on exenatide twice daily to improve HbA1c and body weight without any additional risk for hypoglycemia [11]. In an additional systematic review examining the combined use of insulin with the GLP-1RAs exenatide twice daily, liraglutide (once daily), or lixisenatide (once daily) across 19 studies, the combination consistently resulted in an improvement in HbA1c and body weight [13]. Gastrointestinal AEs, particularly nausea, were the most common AEs but were generally mild and temporary. Furthermore, the authors noted that the convenience of adding a GLP-1RA, which does not require carbohydrate counting or adjustments for meal size, may lead to improved compliance and patient satisfaction.
Other studies of exenatide twice daily treatment in Asian patients have also demonstrated improved glycemic control, without an increased risk for hypoglycemia. In a study of 52 Korean patients, exenatide twice daily treatment was used in patients who were treatment-naïve (group 1), on additional oral glucose-lowering medication (group 2), who were substituting exenatide twice daily for one of their current oral medications (group 3), or adding exenatide twice daily and oral medications to their regimen (group 4) [14]. Over 6 months, all groups reported a significant decrease in HbA1c. Body weight was significantly reduced after 3 months for all groups and after 6 months for all groups except group 4. Nausea was the most common AE and there were no reports of hypoglycemia. In an additional single-arm study of exenatide twice daily, HbA1c and body weight were both significantly reduced among 73 Korean patients with T2DM receiving exenatide twice daily treatment for 22 weeks [15]. The patients did not report any major hypoglycemic events. Placebo-controlled studies conducted in Japanese and Taiwanese populations have also found exenatide twice daily to reduce HbA1c with the additional benefit of weight loss among patients who had inadequate glycemic control with oral glucose-lowering therapy [1617]. These reductions in HbA1c and weight observed with exenatide twice daily were maintained in the long term (52 weeks) in Japanese patients participating in an open-label extension study [18]. Additionally, the findings of a retrospective analysis of pooled data from the exenatide clinical development program support exenatide twice daily as an appropriate injectable treatment option in Asian patients, providing reductions in HbA1c, FG, and PPG, as well as weight loss and low risk of hypoglycemia [19]. Of note, these studies did not utilize basal insulin with exenatide twice daily. As such, this subgroup analysis adds important insight into a new approach to exenatide twice daily therapy among Asian patients.
Limitations of the current analysis are the small sample size and the retrospective nature of the analysis. Therefore, the findings of this study should be considered in conjunction with the results from the overall 4B study population. In conclusion, in this subgroup analysis of Korean patients with T2DM, exenatide twice daily or three times daily mealtime insulin lispro added to insulin glargine each reduced HbA1c. FG and weight tended to decrease with exenatide twice daily and increase with insulin lispro. Four patients receiving exenatide twice daily experienced minor hypoglycemia, and nausea and vomiting were the most common gastrointestinal AEs. These results suggest exenatide twice daily may be appropriate for Korean patients unable to achieve glycemic control with basal insulin plus metformin.
ACKNOWLEDGMENTS
The analysis was supported by AstraZeneca. Mollie Marko, PhD, of inScience Communications, Springer Healthcare (New York, NY, USA), provided medical writing support, which was funded by AstraZeneca.
Notes
CONFLICTS OF INTEREST: K.H.Y has served on advisory boards for AstraZeneca, Eli Lilly, Merck, and Pfizer; has received research support from AstraZeneca, Bayer, Merck, and Takeda; and has received speaker fees from Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Novo Nordisk, and Takeda. E.H. is an employee and stockholder of AstraZeneca. J.H. is an employee of Pharmapace, Inc. and was an employee of Bristol-Myers Squibb at the time of data collection.


