Prediction of Gestational Diabetes Mellitus in Pregnant Korean Women Based on Abdominal Subcutaneous Fat Thickness as Measured by Ultrasonography
Article information
Abstract
Background
This study was performed to verify the correlation between abdominal subcutaneous fat thickness (ASFT) measured by ultrasonography (US) during the first trimester of pregnancy and gestational diabetes mellitus (GDM) of the second trimester in Korean women and to establish a standard of ASFT for predicting GDM.
Methods
A total of 333 singleton pregnant women participated in this study. Their ASFT was measured by US during the 10+6 to 13+6 weeks of pregnancy; then a GDM confirmatory test (100 g oral glucose tolerance test) was conducted during the 24 to 28 week period of pregnancy. Based on the GDM tests, comparative analyses of the ages of the subjects, pre-pregnancy body mass index (BMI), and weight gain during pregnancy were conducted.
Results
The ages of the subjects and weight gains during pregnancy were not correlated to the GDM of the second trimester of pregnancy, but the pre-pregnancy BMIs (22±3.3 kg/m2) and the ASFT (1.9±0.5 cm) measurements between the control group and subjects during the first trimester of pregnancy were found to show significant differences (P<0.001). The cut-off value of the ASFT for predicting GDM was determined to be 2.4 cm (area under the curve=0.90, sensitivity 75.61%, specificity 91.78%, P<0.001). The odds ratio was 2.91 (95% confidence interval, 1.07 to 7.92; P=0.034), which was higher than the 2.4 cm ASFT.
Conclusion
It was determined that ASFT as measured by US during the first trimester of pregnancy can be used to predict the risk of developing GDM during the second trimester of pregnancy and for prognosis.
INTRODUCTION
Gestational diabetes mellitus (GDM) can be defined as glucose intolerance either first developed or found during pregnancy regardless of the degree of symptoms. It is a type of diabetes or impaired glucose tolerance developed during pregnancy and one of the most common medical complications of pregnancy [1]. GDM develops in approximately 3% to 4% of pregnant women, and mothers with GDM have a high risk of developing diabetes after delivery as well as their children who also risk developing obesity. It has been reported that GDM increases the frequency of pre-eclampsia, eclampsia, infectious disease, and the birth rate of large for gestational age fetuses in mothers. It also increases perinatal morbidity by increasing the risk of infantile respiratory distress syndrome, metabolic disorders, and hyperbilirubinemia in fetuses [2]. For the tests to diagnose GDM as a screening test, a 50 g oral glucose tolerance test (OGTT) during the 24 to 28 weeks of pregnancy is utilized. Subjects with blood glucose levels measured 1 hour after oral ingestion of glucose of 140 mg/dL (7.8 mol/L) or more are considered positive for the screening test, and for a confirmatory test, a 100 g OGTT is conducted following 12 hours of fasting [3]. Once GDM is confirmed, depending on the level of fasting blood glucose and post-meal (2-hour) blood glucose, diet therapy and exercise or insulin therapy can be conducted. Through such therapies, GDM-related complications in both mothers and their fetuses can be reduced [4].
The age of a mother, her pre-pregnancy body mass index (BMI), the amount of obesity, family history, and weight gain during pregnancy are GDM-related factors, but among these, obesity is the most serious risk factor [56]. Obesity is known to be highly correlated to abdominal subcutaneous fat thickness (ASFT) [7]. Although computed tomography (CT) is the most credible method for measuring ASFT, it is avoided for pregnant women due to its high cost and X-ray exposure. On the other hand, ultrasonography (US) imaging-based measurement of ASFT is very useful for pregnant subjects because the results are highly correlated to CT and there is no risk of X-ray exposure due to its relatively simple measuring process [8].
In this study, we intended to analyze the correlation between ASFT as measured by US in pregnant subjects during the first trimester of pregnancy in order to suggest an ASFT cut-off value as a predictive factor for GDM during the second trimester of pregnancy.
METHODS
Study design and subjects
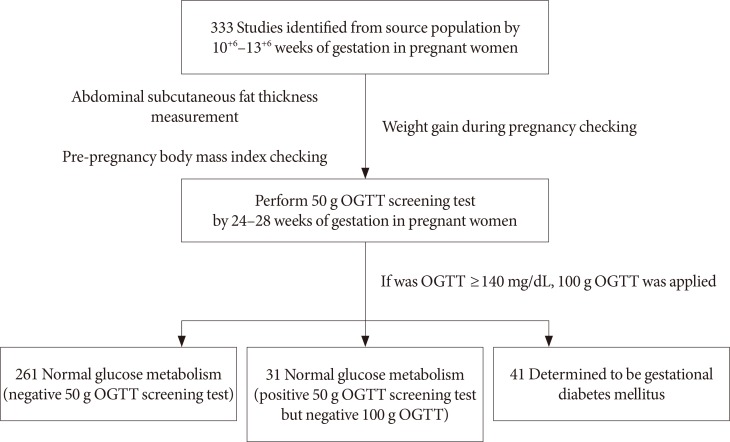
The subjects of this study were 333 singleton pregnant women ages 19 to 41 and in their the first trimester of pregnancy (10+6 to 13+6 weeks) who were under the prenatal care of the Obstetrics and Gynecology Clinic at Ilsin Christian Hospital in South Korea during the period of February 2015 to June 2016. For all subjects, a pre-pregnancy BMI was calculated, and the weight gain during pregnancy was obtained by subtracting the pre-pregnancy weight from the weight measured during the 24 to 28 weeks of pregnancy. The menstrual cycles of both the GDM high-risk group and a control group were shown to be regular, with the last menstrual periods being on time. Subjects having type 1 or type 2 diabetes mellitus, a history of smoking, and/or other medical conditions such as high blood pressure and metabolic syndrome were excluded from the study.
ASFT measurement and blood testing
The ultrasound device was operated by one sonographer using a high-resolution convex array probe (Voluson E8 Expert; GE Healthcare, Chicago, IL, USA) at a frequency of 2 to 8 MHz. The subjects were all placed in a comfortable supine position and then the maximum depth from the skin to the rectus abdominis muscle was measured at 1 cm above the umbilicus using a longitudinal scan [9] at the end of expiration (Fig. 1).
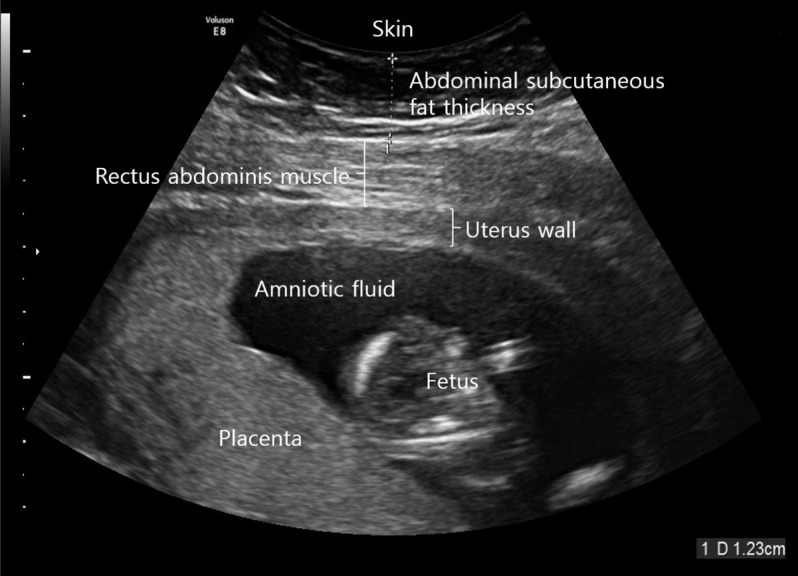
Measuring abdominal subcutaneous fat thickness using a high-resolution ultrasonography at 1 cm above the umbilical level.
The subjects whose ASFT were measured during their 10+6 to 13+6 weeks of pregnancy were subjected to a hexokinase glucose test taken from the veins during their 24- to 28-week period of pregnancy by taking glucose (Diasol-S Solution 50 g; Taejoon Pharm, Seoul, Korea) regardless of eating but fasting for at least 1 hour. If the result of the 50 g OGTT was 140 mg/dL or more, the subject was considered positive and an additional confirmatory OGTT was conducted using 100 g glucose. For this diagnostic test to confirm GDM, the screened positive subjects fasted for 12-hour prior to confirmatory testing with blood collected the next morning. The subjects were administered glucose (Diasol 100 g) orally and then at 1, 2, and 3 hours after taking the glucose, venous blood was collected and the glucose measured. Using the criteria of the national diabetes data group (fasting blood glucose, 105 mg/dL; 1 hour after 100 g Diasol, 190 mL/dL; 2 hours after 100 g Diasol, 165 mg/dL; 3 hours after 100 g Diasol, 145 mg/dL), if the measured values exceeded any two of those criteria, the subject was determined to be GDM (Fig. 2) [10].
Statistical analysis
All data were presented as a mean±standard deviation. To test the mean difference of the baseline characteristics between the GDM high-risk group and the control group, independent sample t-tests were used for both continuous variables and discrete variables, respectively. To determine the cut-off value for predicting GDM, a receiver operating characteristic (ROC) curve analysis was conducted, with the area under the curve (AUC), sensitivity, and specificity calculated. A logistic regression analysis was then conducted to calculate the odds ratio for the ASFT-mediated risk of GDM. MedCalc statistical software version 15.8 (MedCalc Software bvba, Ostend, Belgium) was used for the statistical analysis with a P value of less than 0.05 being considered statistically significant.
RESULTS
General characteristics of test subjects
Among the 333 subjects, 261 (78%) were screened as negative and 72 (22%) were screened as positive during the GDM screening test (50 g OGTT). The number of subjects who initially screened positive but turned out to be negative as a result of the GDM confirmatory test (100 g OGTT) was 31 (9%), with 41 (12%) being determined to be GDM as they were positive for both the screening and confirmatory tests. The average age of the subjects was 32±3.9 (range, 19 to 41) and there was no significant difference among groups (P=0.210). The average pre-pregnancy BMI was 22.0±3.3 kg/m2 (range, 15.7 to 34 kg/m2). According to the Asian standard criteria [11], 37 subjects (11%) were grouped as underweight, 180 (54%) as normal, and 116 (35%) as overweight. Among the 41 subjects diagnosed as having GDM, 23 (26.9±2.6 kg/m2) were deemed overweight (BMI ≥23 kg/m2). This ratio was higher than in the underweight and normal groups (P=0.005). Weight gain during pregnancy was 4.5±2.3 kg (range, 4.5 to 12.0 kg) with no significant difference (P=0.500). ASFT was 1.9±0.5 cm (range, 0.9 to 4.1 cm) with a statistically significant difference (P<0.001) (Table 1).
GDM and ROC curve analysis
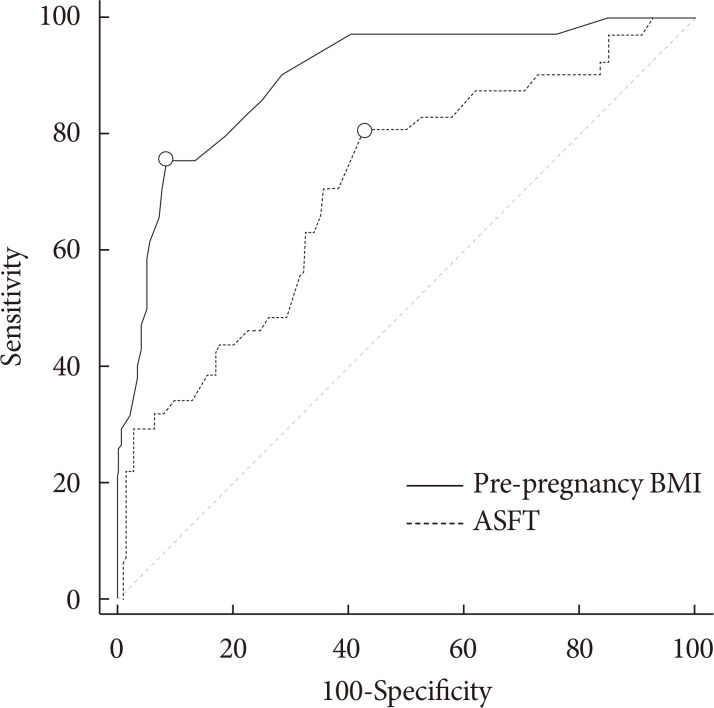
To determine an effective cut-off value for predicting GDM, a ROC analysis was conducted using the AUC of independent variables as accuracy criteria. As a result, we determined that the sensitivity and specificity of the pre-pregnancy BMI were 80.49% and 57.19%, respectively, and determined a cut-off value of 21.8 kg/m2 (AUC=0.71) with a Youden index of 0.377. The sensitivity and specificity of the ASFT were 75.61% and 91.78%, respectively, and the cut-off value was 2.4 cm (AUC=0.90) with a Youden index of 0.674 (Table 2, Fig. 3).
Logistic regression analysis
An elevated ASFT was significantly associated with positive GDM. Using the 2.4 cm ASFT cut-off value, the odds ratio of GDM in 72 pregnant women who underwent the 100 g OGTT was 2.91 (95% confidence interval [CI], 1.07 to 7.92; P=0.034). Even after adjusting for the pre-pregnancy BMI, the odds ratio was 2.96 (95% CI, 0.95 to 9.24; P=0.062) (Table 3).
DISCUSSION
Although the frequency of GDM varies depending on the country, ethnicity, populations screened, and diagnostic criteria, it is commonly reported as 1% to 10% and its incidence rate is generally increasing due to factors such as the increase in maternal age and obesity among mothers. This can further increase the incidence of GDM-related complications and can also cause fatal conditions to fetuses such as dyspnea at birth. For this reason there is presently a dire need to be able to predict and prevent GDM [12]. The most common method for diagnosing GDM is to conduct a 100 g OGTT for those showing blood glucose levels of 140 mg/dL or more in the 50 g OGTT conducted during the 24 to 28 weeks of pregnancy. This test is conducted at the second trimester of pregnancy, so it is difficult to detect GDM at its early stage. Because the incidence rate of obstetric complications varies depending on the time of diagnosis, early detection and active management is required to prevent GDM-related obstetric complications [13].
This study was conducted in the hopes of finding a predictive factor relating to GDM in pregnant Korean women using as independent variables the ages of the mothers, pre-pregnancy BMI, weight gain during pregnancy, and ASFT as measured by ultrasound imaging conducted during the first trimester of pregnancy. To date, studies of the early detection of GDM have been ongoing. A large scale study indicated that pre-pregnancy BMI significantly increases along with the incidence of GDM during the second trimester of pregnancy as the age of a mother increases and when categorized based on ethnicity, the age of a mother and her BMI play more significant roles as risk factors in South Asian and black African women compared to white European or black Caribbean women [14]. Nevertheless, there was no significant correlation between the increased age of the mothers and GDM in this study (P=0.210), and for pre-pregnancy BMI, the incidence of GDM significantly increased as BMI increased from the Asian standard criterion. In particular, the impact was more significant in the overweight group (P=0.005) suggesting the importance of pre-pregnancy weight management for mothers. Further, although Choi [6] reported that the excess weight (or obesity) of a mother or excessive weight gain during pregnancy induced GDM, there was no significant correlation between the weight gain and GDM in this study (P=0.500).
Recently, as numerous metabolic and cardiovascular diseases related to obesity have been observed, methods to accurately evaluate abdominal fat have been gaining attention. Among such methods, despite its drawback of possible measurement error due to interoperator variation, measuring abdominal fat thickness using ultrasound is actively being studied because it can easily be applied to pregnant women and its reproducibility has been reported [15]. The results of a longitudinal cohort study of 1,510 pregnant women in Australia suggested the potential of ASFT as an independent predictor of GDM [16]. The correlation between maternal obesity and GDM has already been reported, and in particular, a study was recently published that examined the relationship between central obesity and the progression of GDM during the second trimester of pregnancy [17].
In a study of Caucasian subjects by De Souza et al. [18], the mean ASFT depth was 1.9±0.80 cm (range, 0.56 to 5.1 cm), consistent with the mean depth of 1.9±0.5 cm (range, 0.9 to 4.1 cm) in this study. However, although De Souza et al. [18] reported that ASFT was not a statistically significant predictor of GDM based on composite outcomes, this study observed a significant difference in GDM according to ASFT as indicated by the significant increase in GDM (P<0.001).
Based on these results, the cut-off value of 2.4 cm (AUC=0.90, P<0.001) was determined and an elevated ASFT was significantly associated with a positive GDM (unadjusted odds ratio, 2.91; 95% CI, 1.07 to 7.92; P=0.034). Therefore, when ASFT at the first trimester of pregnancy is 2.4 cm or more, adequate management is required and care practitioners should be alerted to the possibility of GDM and make efforts to manage diabetes during pregnancy.
One limitation of this study was the inclusion of pregnant women who were admitted to a single general hospital; therefore, these subjects are not representative of the entire population of pregnant women in Korea. Furthermore, various studies have reported different lengths of US-determined visceral fat and subcutaneous fat, and our results did not yield sufficient intraobserver reliability. This suggests the need for standardized measurement methods. In future studies, an identical ASFT measurement method must be applied to a larger population of patients, and this process should be used to standardize the measurement methods.
In conclusion, the results of this study demonstrated that ASFT as measured by ultrasound during the first trimester of pregnancy can be a valuable indicator for predicting GDM during the second trimester. Thus, ASFT, information that can be easily obtained during the first trimester of pregnancy, is expected to be increasingly utilized as an auxiliary diagnostic criterion for predicting the risk of GDM during the second trimester of pregnancy.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.