Gemigliptin: An Update of Its Clinical Use in the Management of Type 2 Diabetes Mellitus
Article information
Abstract
Dipeptidyl peptidase-4 (DPP-4) inhibitors are a new class of oral antidiabetic agent for the treatment of type 2 diabetes mellitus. They increase endogenous levels of incretin hormones, which stimulate glucose-dependent insulin secretion, decrease glucagon secretion, and contribute to reducing postprandial hyperglycemia. Although DPP-4 inhibitors have similar benefits, they can be differentiated in terms of their chemical structure, pharmacology, efficacy and safety profiles, and clinical considerations. Gemigliptin (brand name: Zemiglo), developed by LG Life Sciences, is a potent, selective, competitive, and long acting DPP-4 inhibitor. Various studies have shown that gemigliptin is an optimized DPP-4 inhibitor in terms of efficacy, safety, and patient compliance for treatment of type 2 diabetes mellitus. In this review, we summarize the characteristics of gemigliptin and discuss its potential benefits in clinical practice.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a complex and progressive disease requiring continuous medical care, with multifactorial risk reduction strategies including glycemic control [1]. Therefore, many factors such as adverse events (AEs), disease duration, life expectancy, important comorbidities, and established vascular complications should be taken into account to achieve a patient's individualized goal. Current treatment guidelines recommend the use of dipeptidyl peptidase-4 (DPP-4) inhibitors as first-line therapy or second-line therapy after metformin due to their ease of use, low risk of hypoglycemia, weight neutrality, and favorable tolerability [23]. Currently, there are nine approved DPP-4 inhibitors in Korea including gemigliptin. Although they have some benefits in common through the incretin-based mechanism, DPP-4 inhibitors can be differentiated in terms of pharmacology, efficacy and safety profiles, and clinical considerations along with their different chemical structure [4].
The development program for DPP-4 inhibitors by LG Life Sciences (Seoul, Korea) was initiated in the early 2000s, and gemigliptin was approved by the Ministry of Food and Drug Safety for treatment of T2DM in June 2012 [5]. LG Life Sciences signed a licensing agreement with multinational pharmaceutical companies such as Sanofi (Paris, France) and Stendhal (Mexico City, Mexico) for 104 countries. Currently, gemigliptin has been approved in 11 countries such as India, Columbia, Costa Rica, Panama, and Ecuador, and several clinical studies are in progress in Russia, Mexico, and Thailand.
Gemigliptin is a potent, selective, competitive, and long-acting DPP-4 inhibitor. Various studies have shown that gemigliptin is an optimized DPP-4 inhibitor in terms of efficacy, safety, and patient compliance for treatment of T2DM. In this article, we will review the recent clinical studies of gemigliptin and compare it to the other DPP-4 inhibitors with similarly designed clinical studies. This review includes the recent updates presented in PubMed, presents data for drug approvals by the U.S. Food and Drug Administration and European Medicines Agency, and summarizes the product characteristics and clinical data presented as oral and poster presentations at the American Diabetes Association, European Association for the Study of Diabetes, and International Diabetes Federation congresses.
CHARACTERISTICS OF GEMIGLIPTIN
Rational of drug design
LG Life Sciences aimed to develop a new DPP-4 inhibitor with potent efficacy, high selectivity, and better compliance with once a day (qd) dosing compared to its competitors. At that time, the new compound could be designed based on the analysis of the internally known DPP-4 crystal structure, and the lead compound could be rapidly optimized through structure-based drug design. In particular, the interaction of the active part of the S2 extensive subsite of the DPP-4 enzyme with CF3 groups on the pyrimidine piperidine resulted in an increase of the selectivity and potency of gemigliptin compared with its competitors.
Chemistry
Zemiglo (LG Life Sciences, Seoul, Korea) is the brand name of gemigliptin, which is a novel structure with pyrimidino piperidine derivatives. The co-crystal structure of gemigliptin with the DPP-4 enzyme is shown in Fig. 1. Gemigliptin binds to the S1, S2, and S2 extensive subsites of the DPP-4 enzyme. The piperidinone group of gemigliptin binds to the S1 subsite, where the upside F atom on the piperidin ring forms a hydrogen bond with the side chain of Tyr631 and the downside F atom makes a hydrophobic interaction with the side chain of Tyr666 and Tyr662. In addition, the key interaction occurs between the CF3 groups on the pyrimidino piperidine and the S2 extensive subsite of the DPP-4 substrate, which enhances the potency of the drug and increases its selectivity as well.
In vitro and in vivo pharmacology
Gemigliptin showed the type of reversible and competitive inhibitor with a Ki value of 7.25±0.67 nM. In a kinetic study of interactions between the DPP-4 enzyme and DPP-4 inhibitors, gemigliptin was characterized by a fast association and a slow dissociation, indicating that gemigliptin is a long-acting DPP-4 inhibitor. In addition, gemigliptin showed at least >23,000-fold selectivity for various proteases and peptidase including DPP-8, DPP-9, and fibroblast activation protein-α (Table 1) [67891011121314]. Gemigliptin sustainably inhibited DPP-4 activity in a dose-dependent manner and exerted a more potent DPP-4 inhibitory effect for 24 hours than that of sitagliptin at the same dose in rats, dogs, and monkeys. In in vivo studies, gemigliptin prevents the degradation of active glucagon-like peptide-1 (GLP-1) by DPP-4 inhibition, which results in improvements of glucose tolerance by increasing insulin secretion and reducing glucagon secretion during oral glucose tolerance test. It also decreased in a dose-dependent manner glycosylated hemoglobin (HbA1c) and ameliorated β-cell damage in high-fat diet/streptozotocin-induced diabetic mice [6]. These results suggest that gemigliptin is a potent, selective, and long-acting DPP-4 inhibitor with strong binding to the DPP-4 enzyme.
Recent studies showed that DPP-4 inhibitors exerted pleiotropic effects independent of their glucose-lowering properties [1516]. The potential pleiotropic effects of gemigliptin have also been demonstrated in various disease models. Gemigliptin prevented diabetes-induced podocyte apoptosis and reduced albuminuria in db/db mice [17]. It also prevented the thickening of the diabetes-induced glomerular basement membrane and reduced renal fibrosis in streptozotocin-induced type 1 diabetic mice [18]. In a renal fibrosis model, gemigliptin protected the renal injury via several mechanisms related to fibrosis, inflammation, and oxidative damage [19]. In addition, gemigliptin ameliorated the retinal vascular leakage and loss of tight junction protein in db/db mice and reduced retinal neovascularization in oxygen-induced retinopathy (OIR) mice. These effects were associated with attenuated overexpression of plasminogen activator inhibitor-1, monocyte chemoattractant protein-1, and vascular endothelial growth factor in the retinas of diabetic db/db mice and OIR mice [20]. Gemigliptin also exhibited a potent anti-glycation effect and cardiovascular protective effect in vivo and in vitro studies [2122232425]. Further clinical studies will be needed to elucidate the potential role of gemigliptin for micro- and macrovascular complications.
Pharmacokinetics
The pharmacokinetics of gemigliptin has been extensively characterized in healthy subjects and in patients with T2DM. After oral administration of a 50 mg dose to healthy subjects, gemigliptin was rapidly absorbed, with the maximum plasma concentrations (Cmax) attained at about 1.8 hours. The Cmax and area under the curve (AUC) values were increased in a dose-proportional manner [7]. Following a single oral dose of gemigliptin 50 mg to healthy subjects, the mean plasma AUC of gemigliptin was 743.1 ng/hr/mL, Cmax was 62.7 ng/mL, and apparent terminal half-life was 17.1 hours [8]. Key pharmacokinetic parameters for gemigliptin are summarized in Table 2. In addition, pharmacokinetic studies indicate that gemigliptin does not accumulate with multiple dosing and can be administered with or without food [726].
The elimination of drugs from the body involves the process of metabolism and excretion, and the main routes of excretion generally are via urine and feces. If the clearance mechanism excessively relies onto one of the routes of elimination, or the route is impaired, the plasma concentration can be extremely changed. Therefore, pharmaceutical companies are generally looking for a drug candidate to be eliminated by multiple routes. In a mass balance and excretion study of 14C-gemigliptin in rats, the administered radioactivity was recovered equally in urine (41.2%) and feces (43.6%) [27]. In humans, 90.5% of administered radioactivity of 14C-gemigliptin was recovered: 63.4% from the urine and 27.1% from the feces. Unchanged gemigliptin was the most abundant component in urine and feces, representing 61% (39% of dose) and 41% (11% of dose) of radioactivity in urine and feces, respectively [9]. These results indicated that the elimination route of gemigliptin is relatively balanced between metabolism and excretion through both urine and feces, while other marketed DPP-4 inhibitors are highly depended on one or two elimination pathways [10].
Special populations: renal and hepatic impairment
In an open label, non-randomized, parallel design, a single-dose study was designed to assess the pharmacokinetic profiles of gemigliptin on subjects with renal impairment (RI). In addition, the extent of drug elimination by dialysis was examined in the end-stage renal disease (ESRD) group. The area under the curve from zero to time infinity (AUCinf) of the active moiety showed 1.10, 2.10, 1.99, and 1.98 (2.17) times higher values in mild, moderate, and severe test groups, and ESRD groups on dialysis (non-dialysis period), respectively, than values of the matching control groups. In patients with ESRD, less than 2.9% of the obtained gemigliptin was eliminated through dialysis sessions and there was no significant difference in the pharmacokinetic profiles of gemigliptin between dialysis and non-dialysis periods. Therefore, gemigliptin did not require dose adjustment during the dialysis period [28].
In patients with mild and moderate hepatic insufficiency (according to the Child-Pugh classification), mean AUC of gemigliptin were increased approximately 50% and 80% compared to healthy subjects, respectively. These modest changes, within 2-fold in gemigliptin pharmacokinetics, were not considered to be clinically meaningful. Consequently, gemigliptin has a relatively balanced clearance mechanism, so it is practical for use in the T2DM patient with renal and hepatic impairment without dose adjustment [29].
Drug interactions
In vitro studies indicated that gemigliptin is not an inhibitor of cytochrome P450 (CYP) 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, or 3A4, and it is also not an inducer of CYP1A2, 2C8, 2C9, 2C19, or 3A4. Therefore, gemigliptin is unlikely to cause interactions with other drugs that utilize these metabolic pathways. In vitro studies further indicated that gemigliptin did not induce p-glycoprotein (p-gp), while it mildly inhibited p-gp-mediated transport at a high concentration. Therefore, gemigliptin is unlikely to cause significant interactions with other p-gp substrates at therapeutic concentrations.
In several drug-drug interaction studies, gemigliptin did not meaningfully alter pharmacokinetics of co-medications frequently used to treat T2DM, such as antidiabetic agents (metformin, pioglitazone, and glimepiride), and antihypertensive and lipid-lowering agents (irbesartan and rosuvastatin) [30313233]. Although co-administration of ketoconazole, a potent inhibitors of CYP3A4, resulted in a moderate increase in gemigliptin exposure (1.9-fold as total active moiety of gemigliptin), no dosage adjustment should be required when gemigliptin is co-administered with ketoconazole. In addition, gemigliptin exposure may be reduced when co-administered with rifampicin, a strong CYP3A4 inducer [34].
CLINICAL EFFICACY OF GEMIGLIPTIN
Effects on glycemic control
Monotherapy
The efficacy and safety of gemigliptin monotherapy were evaluated in two double-blind placebo controlled studies and one double-blind active-controlled study. A phase II study (study identifier: LG-DPCL002) of gemigliptin was conducted in a randomized, double-blind, placebo-controlled, parallel group design with three doses of 50, 100, and 200 mg qd for the purpose of finding a dose responsiveness and an optimal dose in patients with T2DM. The mean changes of HbA1c at week 12 from the baseline were –0.98%, –0.74%, –0.78% (when adjusted with placebo data, –0.92%, –0.68%, and –0.72%) at 50, 100, and 200 mg, respectively [35]. Among the effective doses obtained from the phase II study in patients with T2DM, the 50 mg dose showed a similar efficacy as the 100 and 200 mg doses, within the maximum safety margin. Similar findings were reported from two phase III studies. Patients were randomized to receive gemigliptin, either a 50 mg qd (n=90) or a placebo (n=92) for 24 weeks (study identifier: LG-DPCL005; Clinical-Trials.gov registration number: NCT01601990). The placebo-subtracted changes from baseline in HbA1c were reported to be -0.71% (95% confidence interval [CI], -1.04 to -0.37) with gemigliptin 50 mg (Table 3) [35363738394041424344454647484950515253545556575859606162636465666768697071]. In addition, a 28-week open-label extension study was designed to evaluate the long-term safety and efficacy of gemigliptin. Among 165 patients who consented to participate in the extension period of study LG-DPCL005, 158 patients (96%) completed their treatments for 52 weeks. All patients were switched to or continued their treatments only with gemigliptin 50 mg qd during the extension period. A further decrease in HbA1c was observed in the continued treatment with gemigliptin 50 mg in this extension period, and the mean change from baseline at 52 weeks (–0.87%) was still clinically and statistically significant (full analysis set analysis, P<0.0001) [72].

Efficacy of gemigliptin on glycemic control in patients with type 2 diabetes mellitus: summary of data from randomized controlled trials
In another double-blind, active-controlled, phase III trial (study identifier: LG-DPCL011), eligible patients with HbA1c greater than 7.5% were randomized to receive gemigliptin 50 mg qd with metformin slow release (SR) qd (n=141), gemigliptin 50 mg qd (n=142), or metformin SR qd (n=150) for 24 weeks. After 24 weeks, the reduction from the baseline in HbA1c was –1.24% for gemigliptin monotherapy [37].
Initial combination therapy with metformin
The combination therapy of gemigliptin with metformin showed additional effects by increasing the plasma active GLP-1 concentration and lowering the serum glucose level. The plasma glucagon level was also lower in combination therapy with metformin than in monotherapy [32].
In this randomized, double-blind, active-controlled, phase III trial (study identifier: LG-DPCL011, INICOM study; ClinicalTrials.gov registration number: NCT01787396), eligible patients with an HbA1c greater than 7.5% were randomized to gemigliptin 50 mg qd+metformin SR qd (n=141), gemigliptin 50 mg qd (n=142), or metformin SR qd (n=150). From weeks 2 to 6, metformin SR was uptitrated incrementally from 500 to 2,000 mg/day maximum in the gemigliptin/metformin and metformin groups. The mean daily doses of metformin at week 24 were 1,699 and 1,868 mg for the gemigliptin/metformin group and the metformin group, respectively. Mean change in HbA1c from baseline was –2.06% for gemigliptin/metformin group versus –1.24% for the gemigliptin group and –1.47% for the metformin group, respectively (P<0.0001 for all comparisons of combination therapy vs. monotherapy) [37]. The differences in proportions achieving an HbA1c <7% or <6.5% were also statistically significant (P<0.0001) between the combination therapy and the respective monotherapy groups.
Add-on to metformin
A 24-week, multinational, randomized, double-blind, active-controlled study (study identifier: LG-DPCL006; ClinicalTrials.gov registration number: NCT01602003) was designed to assess the efficacy and safety of gemigliptin 50 mg compared to the active control (sitagliptin) added to ongoing metformin therapy in patients with T2DM inadequately controlled with metformin alone (HbA1c, 7% to 11%) [73]. After 24 weeks, the reduction from baseline for HbA1c was 0.81% for gemigliptin 25 mg twice a day (bid) and 0.77% for gemigliptin 50 mg qd, and the differences in the least square mean changes from baseline between groups (each group of gemigliptin-sitagliptin group) were -0.011% in gemigliptin 25 mg bid and 0.004% in gemigliptin 50 mg qd. The proportion of patients achieving an HbA1c <7% at week 24 (gemigliptin 25 mg bid group, 50%; gemigliptin 50 mg qd group, 54.07%) was comparable to the results with sitagliptin 100 mg qd (48.87%). The efficacy of lowering HbA1c in the gemigliptin group was generally consistent across the subgroups based on age (<65 or ≥65 years), gender, duration of T2DM (5, >5 to 10, or >10 years), and baseline body mass index (BMI, <25 or ≥25 kg/m2). In addition, gemigliptin groups led to a significantly greater inhibition of plasma DPP-4 compared to sitagliptin [38].
This study was extended by 28 weeks in order to evaluate the long-term efficacy and safety of gemigliptin. About 90% of the patients who completed 24 weeks of treatment in each group consented to continue the study to receive further treatment with gemigliptin 50 mg qd for 28 weeks. The subjects who had been treated with the active control drug (sitagliptin) were also treated with gemigliptin for the long-term extension periods. All treatment groups showed clinically and statistically (P<0.0001) significant improvement in glycemic control from baseline after 52 weeks. The reduction from the baseline in HbA1c was –1.06 (95% CI, –1.28 to –0.85) in the patients who continued to receive gemigliptin 50 mg qd. When sitagliptin was switched to gemigliptin at week 24, there was additional 0.1% reduction from the baseline in HbA1c at week 52 and significantly more patients receiving gemigliptin (27.3%) than sitagliptin (6.8%) achieved an HbA1c <6.5% [74]. These results show that the switching from sitagliptin to gemigliptin provided the sustained glycemic improvements in patients with T2DM.
Add-on to metformin and glimepiride
In this multicenter, randomized, double-blind, phase III study (study identifier: LG-DPCL010, TROICA study; ClinicalTrials.gov registration number: NCT01990469), eligible patients with inadequate glycemic control (7%≤HbA1c≤11%) were randomized to gemigliptin 50 mg qd (n=109) or placebo (n=110). The baseline demographics were similar between groups (age, 60.9 years; BMI, 24.9 kg/m2; duration of T2DM, 12.9 years), with mean±standard deviation (SD) baseline HbA1c of 8.12%±0.82% in the gemigliptin group and 8.15%±0.89% in the placebo group. At week 24, the adjusted mean±standard error change for HbA1c with gemigliptin was –0.88%±0.17% (change with placebo –0.01%±0.18%; difference –0.87%±0.12%; 95% CI, –1.09 to –0.64; P<0.0001) [39].
Add-on therapy in patients with renal impairment
RI in T2DM limits the usable medications for lowering glucose level and requires frequent monitoring of renal function. Gemigliptin has balanced elimination between urinary/fecal excretion and hepatic metabolism; therefore, it does not require dose adjustment in patient with moderate to severe RI. This study evaluated the efficacy and safety of gemigliptin in T2DM patients with moderate to severe RI. This randomized, double-blind, parallel group, phase IIIb study (study identifier: LG-DPCL015, GUARD study; ClinicalTrials.gov registration number: NCT01968044) was composed of a 12-week, placebo controlled period, followed by a 40-week, double-blind active controlled extension period (placebo switched to linagliptin). A total of 132 patients with moderate or severe RI were randomized to receive gemigliptin (n=66) or placebo (n=66). Insulin was used as predominant background therapy (63.1%). At week 12, the placebo-adjusted mean change in HbA1c from the baseline was –1.20% (95% CI, –1.53 to –0.87; P<0.0001) [40]. A similar profile was also observed in other glycemic control parameters (fasting plasma glucose, glycated albumin, and fructosamine).
Head-to-head comparisons between gemigliptin and other DPP-4 inhibitors
The efficacy and safety of gemigliptin was directly compared with other DPP-4 inhibitors in the three multicenter, randomized, active-controlled studies. Two head-to-head studies between gemigliptin and sitagliptin were conducted in patients with T2DM as an add-on to metformin and initial combination therapy with metformin. As mentioned above, the phase III study showed non-inferiority in HbA1c reduction for gemigliptin compared with sitagliptin in the add-on to metformin therapy [73]. The second study was performed to assess the efficacy of gemigliptin in combination with metformin for the initial treatment of drug-naïve patients with an HbA1c greater than 7.5% (study identifier: LG-DPCL012). After 12 weeks, the mean HbA1c was reduced from baseline by 2.75%, 2.24%, and 2.75% for gemigliptin 50, sitagliptin 100, and glimepiride 2 mg qd, respectively. However, there was no significant difference in HbA1c reduction between groups [75].
The other head-to-head study (study identifier: LG-DPCL015, GUARD study; ClinicalTrials.gov registration number: NCT01968044) that compared gemigliptin and linagliptin was performed in the T2DM patients with RI. As mentioned above, the placebo group was switched to linagliptin 5 mg qd during the 40-week extension period. After 52 weeks, the adjusted mean±standard error change from baseline in HbA1c was –1.00%±0.21% and –0.65%±0.22% in the gemigliptin and placebo/linagliptin groups, respectively [76]. During the 40-week extension, AEs were reported in 68.0% and 73.1% of subjects using gemigliptin and linagliptin, respectively. The incidence of hypoglycemia was similar between treatment groups (gemigliptin, 20.0%; linagliptin, 28.8%). There were no meaningful changes from the baseline in the body weight (gemigliptin, 0.28 kg; linagliptin 0.33 kg).
Effects on glycemic variability
Glycemic variability and chronic sustained hyperglycemia are the main components of dysglycemia in diabetes. The previous studies suggested that different pharmacodynamic profiles between DPP-4 inhibitors have been associated with the different effects on glycemic variability [7778]. In this study, a multicenter, randomized, active-controlled, parallel group, open-label, exploratory study was designed to evaluate the efficacy on glycemic variability and safety of initial combination therapy of gemigliptin 50 mg qd versus sitagliptin 100 mg qd, or glimepiride 2 mg qd with metformin in patients with T2DM (study identifier: LG-DPCL012, STABLE study; ClinicalTrials.gov registration number: NCT01890629). The mean amplitude of glycemic excursions (MAGE) and SD of glucose were used for assessing glucose fluctuations from the baseline after 12 weeks of treatment. At 12 weeks, MAGE was significantly lower in the DPP-4 inhibitor groups (gemigliptin and sitagliptin) than in the glimepiride group (–43.1, –38.3, and –21.7 mg/dL, respectively). Furthermore, the SD of mean glucose was significantly lower in patients with gemigliptin when compared with sitagliptin (P=0.023) and glimepiride (P=0.0058) [75].
Beyond glucose control
Effect on lipid profiles
In addition to improvements in glycemic control, gemigliptin as mono- and combination therapy slightly decreased mean total cholesterol, low density lipoprotein cholesterol, and triglycerides. The result was summarized in Table 4.
Effect on albuminuria
The GUARD study also evaluated whether gemigliptin had renoprotective effects in type 2 diabetic patients with moderate to severe RI. The urinary albumin creatinine ratio mean changes from baseline were –339 mg/g Cr with gemigliptin compared with 172 mg/g Cr with the placebo (P<0.0001) after 12 weeks. This reduction in albuminuria of the gemigliptin group was observed regardless of glucose lowering effect, blood pressure change, and the use of a renin-angiotensin-aldosterone system blocking agent. Furthermore, urinary nephrin in the gemigliptin group were also significantly reduced compared to the placebo group [76].
Ongoing studies
Several clinical studies in LG Life Sciences are actively underway to assess the efficacy and safety as an add-on combination therapy with insulin (with or without metformin) (ClinicalTrials.gov registration number: NCT02831361), to evaluate the efficacy and safety of gemigliptin-rosuvastatin fixed-dose combination in patients with T2DM and dyslipidemia in phase III clinical trials (ClinicalTrials.gov registration number: NCT02126358), and to evaluate the efficacy and safety of gemigliptin compared with vildagliptin in Russian patients with T2DM (ClinicalTrials.gov registration number: NCT02343926).
Safety and tolerability of gemigliptin
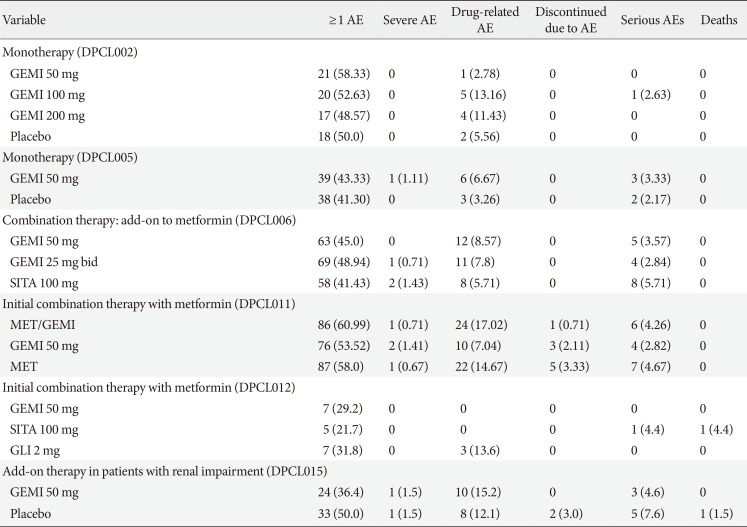
A pooled safety analysis was performed with subjects who had received the investigational product at least once in studies LG-DPCL002, LG-DPCL005, LG-DPCL006, LG-DPCL010, LG-DPCL011, and LG-DPCL012. In the safety analysis, 1,080 patients received an oral dose of gemigliptin 50 mg in the comparative clinical studies, and 446 patients received other oral antidiabetic agents (OADs). The most commonly reported AEs were upper respiratory tract infection, urinary tract infection, nasopharyngitis, headache, diarrhea, arthralgia, hypertension, and cough. Nasopharyngitis was the only AE reported in more than 5.0% of subjects, and its incidence rate was similar between the two groups, with 6.7% and 6.3% in gemigliptin and other OADs groups, respectively. Table 5 shows the frequency of AEs and discontinuation of treatment due to AEs from clinical trials of gemigliptin. The incidence of hypoglycemia was similar to placebo or active control groups when gemigliptin was administered as monotherapy or combination therapy. Most events were mild to moderate in nature and did not require an additional treatment or discontinuation. In addition, treatment with gemigliptin does not increase body weight (Table 6).
Overall, gemigliptin was generally well tolerated in controlled clinical studies in both monotherapy and combination therapy. The incidences of AEs were generally similar to incidences in the placebo and active control groups.
CONCLUSIONS
Based on the findings from the studies in this review, Table 7 summarizes key points contained in the article. Gemigliptin is a potent, selective, and long-acting DPP-4 inhibitor and has been shown to be effective and well tolerated as monotherapy and combination therapy in patients with T2DM. In addition, gemigliptin is more effective in reducing glycemic variability than glimepiride and sitagliptin, and lowers albuminuria independent of its glucose lowering effect. Therefore, gemigliptin may provide important benefits for special populations, especially patients with renal insufficiency and/or the elderly. The results from ongoing clinical studies will also provide additional evidence of the clinical benefits of gemigliptin in the management of T2DM.
Notes
CONFLICTS OF INTEREST: S.H.K. and J. H.Y. are employees of LG Life Sciences, Ltd.