The Efficacy and Safety of Moderate-Intensity Rosuvastatin with Ezetimibe versus High-Intensity Rosuvastatin in High Atherosclerotic Cardiovascular Disease Risk Patients with Type 2 Diabetes Mellitus: A Randomized, Multicenter, Open, Parallel, Phase 4 Study
Article information
Abstract
Background
To investigate the efficacy and safety of moderate-intensity rosuvastatin/ezetimibe combination compared to highintensity rosuvastatin in high atherosclerotic cardiovascular disease (ASCVD) risk patients with type 2 diabetes mellitus (T2DM).
Methods
This study was a randomized, multicenter, open, parallel phase 4 study, and enrolled T2DM subjects with an estimated 10-year ASCVD risk ≥7.5%. The primary endpoint was the low-density lipoprotein cholesterol (LDL-C) change rate after 24-week rosuvastatin 10 mg/ezetimibe 10 mg treatment was non-inferior to that of rosuvastatin 20 mg. The achievement proportion of 10-year ASCVD risk <7.5% or comprehensive lipid target (LDL-C <70 mg/dL, non-high-density lipoprotein cholesterol <100 mg/dL, and apolipoprotein B <80 mg/dL) without discontinuation, and several metabolic parameters were explored as secondary endpoints.
Results
A hundred and six participants were assigned to each group. Both groups showed significant reduction in % change of LDL-C from baseline at week 24 (–63.90±6.89 vs. –55.44±6.85, combination vs. monotherapy, p=0.0378; respectively), but the combination treatment was superior to high-intensity monotherapy in LDL-C change (%) from baseline (least square [LS] mean difference, –8.47; 95% confidence interval, –16.44 to –0.49; p=0.0378). The combination treatment showed a higher proportion of achieved comprehensive lipid targets rather than monotherapy (85.36% vs. 62.22% in monotherapy, p=0.015). The ezetimibe combination significantly improved homeostasis model assessment of β-cell function even without A1c changes (LS mean difference, 17.13; p=0.0185).
Conclusion
In high ASCVD risk patients with T2DM, the combination of moderate-intensity rosuvastatin and ezetimibe was not only non-inferior but also superior to improving dyslipidemia with additional benefits compared to high-intensity rosuvastatin monotherapy.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) and dyslipidemia are significant risk factors for cardiovascular disease, such as coronary heart disease, stroke, and peripheral artery disease, leading to increased mortality and poorer quality of life [1,2]. Therefore, strict control of atherogenic low-density lipoprotein cholesterol (LDL-C) is recommended for all patients with diabetes, and statin therapy has been the primary therapeutic agent for such patients. In recent years, dyslipidemia guidelines have placed a greater emphasis on strict LDL-C control for patients with diabetes. For patients with high LDL-C, moderate-intensity statins were recommended, whereas more potent statins were required for those with an estimated 10-year atherosclerotic cardiovascular disease (ASCVD) risk of more than 7.5% [3]. In 2018, the American Heart Association/American College of Cardiology guideline revised this to include high ASCVD risk as more than 20% [4]. However, concerns remain about the use of highintensity statin therapy, especially in Asian patients, including Koreans. Although lipid-lowering efficacy is more pronounced in Asians than in Caucasians, side effects or adverse events are more frequent in Asian individuals [5-7].
Recently, combination therapy with ezetimibe and statins has shown clinically significant reductions in LDL-C levels and tolerability compared to a higher dose of statins [8,9]. A longterm study found that moderate-intensity statin and ezetimibe was not inferior to high-dose statin therapy in major adverse cardiovascular event (MACE) outcomes and LDL-C reduction in Korean patient with ASCVD [10]. However, there are only a few studies comparing moderate-intensity statin with ezetimibe therapy and high-dose statin therapy in T2DM patients with ASCVD risk.
Therefore, the key question of this trial was whether moderate-intensity statin with ezetimibe combination is replaceable the high-intensity statin therapy. The aim of this trial was to investigate the efficacy and safety of rosuvastatin and ezetimibe 10/10 mg combination therapy compared to rosuvastatin 20 mg monotherapy in T2DM patients with ASCVD risk (≥7.5%).
METHODS
Subjects
Subjects who were in 40 to 75 years old, with T2DM, estimated 10-year ASCVD risk ≥7.5%, body mass index (BMI) less than 35 kg/m2 and glycosylated hemoglobin (HbA1c) levels between 6% and 10% were eligible to participate. Key exclusion criteria were type 1 diabetes mellitus, renal failure (Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] estimated glomerular filtration rate <30 mL/min/1.73 m2 or on renal replacement therapy such as hemodialysis or peritoneal dialysis), impaired liver function (aspartate transaminase [AST], alanine transaminase [ALT] more than three times of upper normal limit), history of taking other statins rather than rosuvastatin within 3-month prior to screening, thiazolidinediones and fenofibrate were excluded. Full list of inclusion and exclusion criteria were provided in Appendix 1. The trial was done in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by Institutional Review Board Yeungnam University Hospital (YUH-2017-08-022) at each trial sites. All participants provided written, informed consent before participating the trial.
Procedures
This study was phase 4, multicenter, open, randomized, parallel study conducted at Republic of Korea for 24 weeks (ClinicalTrials. gov NCT03403556). Total 106 subjects were enrolled from March 7, 2018 to February 14, 2022. After a 2-week of screening period, eligible subjects were randomized in 1:1 ratio to either oral rosuvastatin 20 mg or rosuvastatin 10 mg/ezetimibe 10 mg, prescribed once daily. Randomization was stratified by HbA1c level (less than 9% or more than 9%) at the time of participants visit for screening.
Study endpoints
The primary endpoint was the LDL-C change rate after 24-week rosuvastatin/ezetimibe 10/10 mg treatment was non-inferior to that of rosuvastatin 20 mg. The key secondary endpoint was proportion of subjects who achieved the comprehensive lipid target without dropout due to adverse events, which was defined as LDL-C <70 mg/dL, non-high-density lipoprotein cholesterol (non-HDL-C) <100 mg/dL, and apolipoprotein B (ApoB) <80 mg/dL. Changes of lipid profiles or glycemic parameters such as HbA1c, homeostasis model assessment of insulin resistance (HOMA-IR), and homeostasis model assessment of β-cell function (HOMA-β) at week 24 were also analyzed. Other secondary endpoints were provided in Appendix 2.
The safety endpoints included treatment emergent adverse event, adverse drug reaction (ADR), serious adverse event, serious ADR, and adverse events of special interest that occurred during the trial period. Laboratory evaluations included hematology, blood chemistry which include AST, ALT, creatine phosphokinase, and electrocardiography for both group during the trial period.
Statistical analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Categorical data were presented with frequency (number) and percentage (%) by category, and continuous data were presented with descriptive statistics (number of subjects, mean, standard deviation, median, minimum, maximum). All tests performed for the significance of inter-group difference, intra-group efficacy analysis before and after treatment, and safety assessments were to be two-sided at a significance level of 5%. Difference between groups in percent change (%) from baseline in LDL-C at week 24 was tested using analysis of covariance (ANCOVA) with baseline LDL-C value and HbA1c level (<9%, ≥9%) as covariates; least square (LS) mean and standard error (SE) for each treatment group as well as inter-group difference in LS mean and its 95% confidence interval (CI) with P value were presented. Safety outcome analysis for comparison of adverse event rate between two groups was conducted through chi-square test or Fisher’s exact test.
RESULTS
Baseline characteristics of participants
The study flow is presented in Supplementary Fig. 1. Of 106 subjects, 94 (88.6%) subjects completed the 24 weeks of the study and 12 (11.3%) subjects were withdrawal (three in the rosuvastatin group and nine in the rosuvastatin/ezetimibe group). Demographic and clinical baseline characteristics were similar between two groups (Table 1). The mean age of the subjects was 61 years, male was 72.55% in rosuvastatin group and 58.33% in rosuvastatin 10 mg/ezetimibe 10 mg group. The mean BMI was 25 kg/m2, the mean duration of diabetes were 102.66 months in rosuvastatin 20 mg group and 117.01 in rosuvastatin 10 mg/ezetimibe 10 mg group, the mean HbA1c (%) was 7.39% and the mean LDL-C was 114.34 in rosuvastatin 20 mg group and 121.04 in rosuvastatin 10 mg/ezetimibe 10 mg group.
Primary endpoints
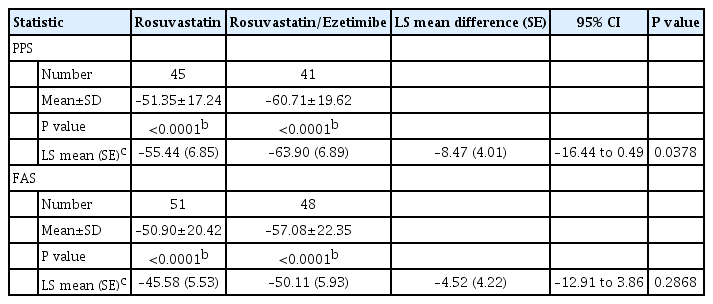
Both rosuvastatin/ezetimibe 10 mg/10 mg and rosuvastatin 20 mg significantly reduced rate change of LDL-C from baseline at week 24 in the per-protocol analysis (–63.90%±6.89% vs. –55.44%±6.85% from baseline, P<0.0001; respectively). In addition, the combination treatment was superior to high-intensity monotherapy in LDL-C change (%) from baseline (LS mean difference, –8.47±4.01; 95% CI, –16.44 to –0.49; P=0.0378) (Table 2). In full analysis set (FAS) analysis, both groups consistently showed significant reduction in LDL-C change rate at week 24 (–50.11±5.93 vs. –45.58±5.53, combination vs. rosuvastatin 20 mg, P<0.0001; respectively) but no statistical difference between two groups (LS mean difference, –4.52±4.22; 95% CI, –12.91 to –3.86; P=0.2868) (Fig. 1).
Secondary endpoints
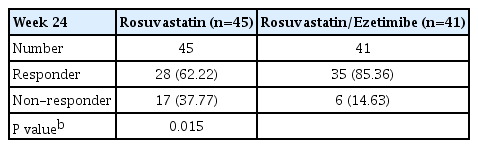
Regarding achievement rate of comprehensive lipid targets (LDL-C <70 mg/dL, non-HDL-C <100 mg/dL, and ApoB <80 mg/dL), higher proportion of combination group achieved rather than monotherapy group at week 24 (85.36% in combination therapy vs. 62.22% in monotherapy, P=0.015) (Table 3).
The LS mean (SE) reduction of calculated LDL-C for perprotocol set (PPS) at week 24 was –72.92 (8.34) in rosuvastatin group and, –82.78 (8.37) in rosuvastatin/ezetimibe group. The LS mean difference between two groups showed significant difference (95% CI, –19.59 to –0.13; P=0.0472). However, there was no significant difference between two group at FAS. Other lipid profiles including triglyceride, non-HDL-C, and ApoB were decreased in each group after 24-week treatment but did not show a significant difference between groups in both PPS and FAS analysis (Fig. 2A).

Secondary endpoints. (A) Changes of lipid profiles after 24 weeks. (B) Changes of homeostatic model assessment index. LDL-C, low-density lipoprotein cholesterol; LS, least square; R, rosuvastatin 20 mg; R/E, rosuvastatin 10 mg and ezetimibe 10 mg; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; ApoB, apolipoprotein B; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, homeostasis model assessment of β-cell function. aP<0.05.
Interestingly, in case of HOMA-β, combination group showed higher increase in HOMA-β than monotherapy. The mean change (SE) of rosuvastatin/ezetimibe 10 mg/10 mg was 11.51 (12.28) and significantly higher than that of rosuvastatin 20 mg, which was –5.63 (12.12) (LS mean difference, 17.13; 95% CI, 2.95 to 31.31; P=0.0185) (Fig. 2B). But there was no significant difference in HOMA-IR and other metabolic indexes between two treatment group (Supplementary Table 1).
Safety outcomes
During the trial periods, several adverse events were reported in both group and these were summarized in Table 4. Most adverse events were mild to moderate in both groups. Total 4 ADRs were reported, 1 (1.92%) in rosuvastatin group and 3 (6.12%) in rosuvastatin/ezetimibe group. Two participants discontinued the trial because of these events in rosuvastatin/ezetimibe group; however, both events were reported as mild case. Overall, there was no significant difference between two groups in safety outcome.
DISCUSSION
In this trial, a moderate dose of rosuvastatin combined with ezetimibe was not only comparable but also superior in lowering LDL-C efficacy in individuals with T2DM and high ASCVD risk. The combination therapy resulted in a higher proportion of achieving comprehensive lipid targets and a greater reduction in calculated LDL-C levels. Interestingly, the combination treatment group also showed an improvement in HOMA-β compared to the monotherapy group at 24 weeks. Rosuvastatin/ezetimibe 10 mg/10 mg was a safe treatment option that did not increase adverse events, such as hepatoxicity or myopathy.
Previous studies have demonstrated that combination therapy with ezetimibe and a moderate-intensity statin is non-inferior to high-intensity statin therapy in terms of lowering LDL-C. In fact, several studies have shown that combination therapy is superior to high-intensity statin therapy in reducing LDL-C levels [11-13]. A single-center randomized controlled trial conducted in Korea found that low-dose rosuvastatin (5 mg) in combination with ezetimibe was not inferior to high-dose rosuvastatin (20 mg) in reducing LDL-C levels in patients with T2DM. However, this trial did not evaluate ASCVD risk [11]. Another randomized controlled trial conducted in Korea found that rosuvastatin/ezetimibe 10 mg/10 mg was superior to rosuvastatin 20 mg after 8 weeks of treatment, with similar potency of LDL-C reduction to our trial [13]. However, this trial included only a few patients with T2DM, which limits its ability to assess the efficacy of combination therapy in this population. In our 24-week study, all participants had diabetes with moderate to high ASCVD risk. Our results confirmed the benefits of combination therapy for people at higher risk.
In terms of secondary efficacy outcomes, our trial demonstrated similar results in achieving comprehensive lipid targets as previous studies [11-13]. However, we also observed an unexpected improvement in HOMA-β as an insulin secretory surrogate marker in the ezetimibe combination group, while it decreased in the high-dose statin group. This finding is noteworthy in light of previous research that has raised concerns about the dose-dependent risk of glucose intolerance and newonset diabetes associated with statin therapy [14]. Furthermore, a study based on the Korean population database found that statin use increased the incidence of new-onset diabetes in Korean patients with dyslipidemia [15]. These observations are linked to insulin resistance, which can lead to the development of diabetes [16,17]. However, experimental studies have shown that ezetimibe can improve insulin secretory function and insulin resistance, particularly by protecting β-cells from glucotoxicity via CD36 inhibition [18,19]. While we did not observe any deterioration in fasting glucose, HbA1c, or HOMA-IR in the high-intensity group over the 6-month period, our findings suggest that the ezetimibe combination may offer lipid-lowering options with additive benefits for patients with diabetes.
Lower dose statin therapy in combination with ezetimibe was hypothesized as a safe alternative to address the dose-related side effects of statins. A meta-analysis that included 22 randomized controlled trials showed that, compared to placebo, a relatively low-dose of statin did not result in differences in discontinuation rates or myopathy [20]. Consistent with these findings, all adverse events observed in the present study were not severe, and there was no significant difference in safety outcomes between the two treatment groups. Thus, both agents are considered safe options. However, combination treatment with ezetimibe may be preferred by individuals with diabetes who are at a higher risk of experiencing side effects from higher dose statins.
Our study has some limitations. The sample size was relatively small, and the study only included Korean patients. In addition, the trial only assessed the efficacy of lowering LDL-C and did not assess MACE outcomes, so we could not compare which therapy had more cardiovascular benefits, which is of high clinical significance. The RACING trial used the same dose of statin and ezetimibe were used as in this study and showed that the combination of low-dose statin and ezetimibe was non-inferior to high-dose statin in terms of MACE outcomes [10]. Finally, due to the relatively short study duration, further long-term studies are needed to confirm our findings.
Despite these limitations, our study also has several strengths. First, all participants were patients with diabetes, and our study suggests the efficacy and safety of rosuvastatin plus ezetimibe in T2DM patients at high risk of cardiovascular disease. Second, we analyzed glucose metabolic parameters such as A1c, HOMA-β and HOMA-IR as secondary endpoints. High-dose statins such as rosuvastatin 20 mg or atorvastatin 40 mg have concerns about increasing the risk of diabetes or worsening glycemic control [21,22], but moderate rosuvastatin with ezetimibe did not deteriorate the glycemic control. In addition, we assessed glycemic parameters, including the insulin secretory function index (HOMA-β), which revealed the potential benefits of the ezetimibe combination for patients with diabetes. Overall, our study provides important insights into the use of combination therapy for treating dyslipidemia in patients with diabetes and highlights the need for further research in this area.
In conclusion, among moderate and high ASCVD risk patients with T2DM, the combination of moderate-intensity rosuvastatin and ezetimibe was not only non-inferior but also superior to improving dyslipidemia with additional benefits compared to high-intensity rosuvastatin monotherapy.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2023.0171.
Secondary efficacy outcomes (per-protocol set): changea from baseline at week 24
Patient disposition.
Notes
CONFLICTS OF INTEREST
Kyu Chang Won has been honorary editor of the Diabetes & Metabolism Journal since 2020. Jun Sung Moon has been associate editor of the Diabetes & Metabolism Journal since 2022. Hye Soon Kim has been associate editor of the Diabetes & Metabolism Journal since 2022. Seung Hyun Ko has been executive editor of the Diabetes & Metabolism Journal since 2022. They were not involved in the review process of this article. Otherwise, there was no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception or design: J.S.M., N.H.K., S.G.K., K.C.W.
Acquisition, analysis, or interpretation of data: J.S.M., S.S.K., H.S.K., N.H.K., S.G.K., S.H.K., J.H.L., I.L., B.K.L., K.C.W.
Drafting the work or revising: I.R.P., J.S.M., B.K.L., K.C.W.
Final approval of the manuscript: J.S.M., I.R.P., S.S.K., N.H.K., S.G.K., S.H.K., J.H.L., I.L., B.K.L., K.C.W.
FUNDING
This study was supported by Yuhan Co. Ltd., Seoul, Republic of Korea. The sponsor participated in the study design, data collection, and analysis of the data. The sponsor had no role in writing the manuscript and in the decision to submit the manuscript for publication.
Acknowledgements
None