Type 2 Diabetes Mellitus and Sarcopenia as Comorbid Chronic Diseases in Older Adults: Established and Emerging Treatments and Therapies
Article information
Abstract
Type 2 diabetes mellitus (T2DM) and sarcopenia (low skeletal muscle mass and function) share a bidirectional relationship. The prevalence of these diseases increases with age and they share common risk factors. Skeletal muscle fat infiltration, commonly referred to as myosteatosis, may be a major contributor to both T2DM and sarcopenia in older adults via independent effects on insulin resistance and muscle health. Many strategies to manage T2DM result in energy restriction and subsequent weight loss, and this can lead to significant declines in muscle mass in the absence of resistance exercise, which is also a first-line treatment for sarcopenia. In this review, we highlight recent evidence on established treatments and emerging therapies targeting weight loss and muscle mass and function improvements in older adults with, or at risk of, T2DM and/or sarcopenia. This includes dietary, physical activity and exercise interventions, new generation incretin-based agonists and myostatin-based antagonists, and endoscopic bariatric therapies. We also highlight how digital health technologies and health literacy interventions can increase uptake of, and adherence to, established and emerging treatments and therapies in older adults with T2DM and/or sarcopenia.
INTRODUCTION
Globally, almost 500 million individuals have type 2 diabetes mellitus (T2DM) and prevalence is increasing [1]. Most adults with T2DM have overweight or obesity [2] and the hallmark characteristic of this disease, insulin resistance, leads to vascular complications and subsequent adverse outcomes [3,4]. T2DM is most prevalent in older adults [1] and associated with age-related conditions, including the skeletal muscle disease sarcopenia [5-7]. Sarcopenia is characterised as the age-related decline in skeletal muscle mass and function [8-10], and 10% to 27% of older adults have this condition [11]. Sarcopenia is associated with adverse outcomes including higher risk of falls, fractures, and premature mortality [12-14]. Sarcopenia has a bidirectional relationship with T2DM as components of both diseases worsen each other via positive feedback loops [5-7]. This review explores recent evidence on the relationship between T2DM and sarcopenia, including established and emerging treatments for managing these diseases, individually or concurrently.
OPERATIONAL DEFINITIONS OF SARCOPENIA
There are several operational definitions of sarcopenia (Table 1) [8-10], resulting in inconsistent prevalence estimates and associations between sarcopenia and adverse outcomes. The most widely adopted is the revised European Working Group on Sarcopenia in Older People (EWGSOP) definition. The EWGSOP diagnoses sarcopenia as the presence of low muscle strength and mass, and determines severity based on physical performance [8]. Recently, the Australian and New Zealand Society for Sarcopenia and Frailty Research (ANZSSFR) published a Delphi method-based consensus statement supporting the use of this definition in Australia and New Zealand [15]. The Asian Working Group for Sarcopenia’s (AWGS) revised definition requires low muscle mass in the presence of low muscle strength or poor physical performance to diagnose sarcopenia, with the presence of all three identifying severe cases [9]. The Sarcopenia Definitions and Outcomes Consortium (SDOC) [10] used statistical procedures to identify cut-points for sarcopenia based on clinical outcomes, resulting in the omission of low muscle mass as a requisite for the diagnosis of sarcopenia, and requiring only low muscle (hand grip) strength and poor physical performance (slow gait speed). The SDOC reported that low relative strength (i.e., low muscle strength scaled to measures of body size), was more consistently predictive of clinical outcomes compared with absolute muscle strength [12]. Overweight and obesity based on body mass index (BMI) are associated with higher absolute, but lower relative, muscle mass and strength [16-18], and it may therefore be appropriate to utilise relative muscle cut-points in populations with T2DM to ensure these individuals receive appropriate access to sarcopenia management interventions.
SARCOPENIA AND TYPE 2 DIABETES MELLITUS
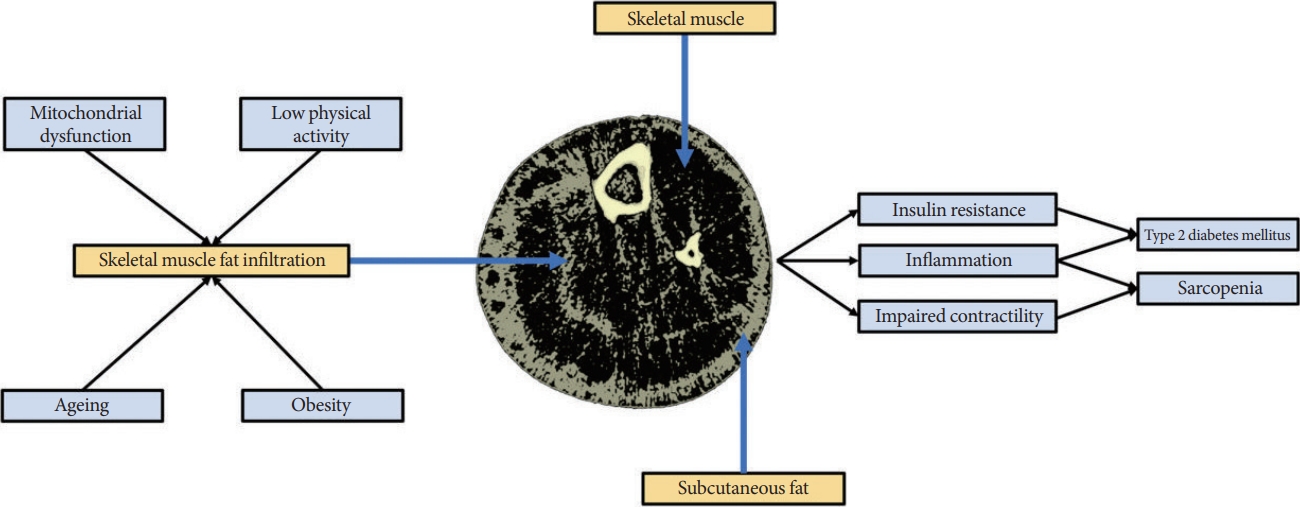
A link between T2DM and sarcopenia has been reported in several studies [19-22]. A recent cross-sectional study in 6,381 adults aged >50 years reported that the prevalence of sarcopenia in those with T2DM was 28% versus 16% in counterparts without T2DM [19]. Sarcopenia is also associated with two-fold higher odds of having diabetes (any subtype) [20]. Meta-analyses have identified associations between characteristics or components of T2DM and sarcopenia. Increased glycosylated hemoglobin (HbA1c) and the presence of vascular complications have been associated with increased odds for sarcopenia (1.2-fold and 2.4-fold, respectively) in people with diabetes [20,23], while those with T2DM have lower muscle strength and physical performance compared to counterparts without T2DM [21]. To date, few longitudinal studies have assessed the relationship between these diseases. The English Longitudinal Study of Ageing, including 5,953 older adults (mean age, 63 years), reported risk for incident T2DM over 6 years decreased by 2% for every 1-kg increase in grip strength [22]. In 824 Japanese older adults, individuals with T2DM had 2.5-fold higher odds of developing sarcopenia over 5 years compared to counterparts without T2DM [24]. Further longitudinal studies are required to clarify causality in what appears to be a bidirectional relationship, particularly since T2DM and sarcopenia share common risk factors including low physical activity, malnutrition, inflammation, mitochondrial dysfunction [25,26]. Myosteatosis may be a key mechanism linking many of these risk factors to the development of both sarcopenia and T2DM.
MYOSTEATOSIS
‘Myosteatosis’ describes adipose depots within skeletal muscle, including intermuscular adipose tissue (IMAT), extracellular adipose tissue found beneath the fascia and in-between muscle groups (known as intramuscular adipose tissue), and intramyocellular lipids (IMCLs) [27]. Myosteatosis, which increases with age and is caused by factors including cellular senescence, obesity, lower physical activity and mitochondrial dysfunction, is a key risk factor for both T2DM and sarcopenia and may mediate their bidirectional relationship [27,28]. Studies have shown that both IMAT and specific IMCL species are positively associated with skeletal muscle insulin resistance [29-35], a key defect in T2DM [36]. The speculated mechanisms explaining the link between myosteatosis and insulin resistance are complex and described elsewhere [37-41]. For example, the ‘athlete’s paradox’, describes the phenomenon whereby endurance-trained athletes have significantly higher IMCLs/diacylglycerol content compared with obese or lean sedentary counterparts, yet are markedly insulin-sensitive [37,38].
Although higher muscle lipid content is a beneficial adaptation in trained endurance athletes, higher IMAT is associated with poorer physical performance in older adults [42-45], which appears to be independent of muscle mass [43]. IMAT also appears to be a stronger predictor of mobility than muscle mass [42] and is associated with higher fall risk in older adults [46,47]. Muscle health may be adversely affected by IMAT through increased localised inflammation and impaired contractility [29,48], but further research is required to obtain additional mechanistic insights into these relationships. The links between myosteatosis, fatty acid (FA) metabolism, insulin resistance and muscle function may be crucial for understanding the etiology of, and relationship between, T2DM and sarcopenia (Fig. 1).
INTERVENTIONS FOR IMPROVING METABOLIC AND MUSCLE HEALTH
Diet and nutrition
Evidence-based dietary recommendations are available for the prevention and management of T2DM and sarcopenia. However, recommendations for these diseases can at times be conflicting, especially in older adults. Dietary recommendations are discussed below with consideration of how these can be implemented in older adults who have T2DM and/or sarcopenia.
Dietary approaches for weight loss
Weight loss achieved through lifestyle changes (i.e., diet and exercise) is a first-line treatment for T2DM in adults with overweight or obesity (BMI ≥25 kg/m2) [49]. However, the appropriateness of using BMI cut-offs for older adults aged ≥65 years has been disputed, as emerging evidence has suggested that a higher BMI may be protective against mortality in this population (the “obesity paradox”) [50]. This is supported by a meta-analysis (n=197,940; follow-up 5 years) showing that older adults with BMI between 24.0 and 30.9 kg/m2 had the lowest mortality risk [51]. This should be considered when prescribing weight loss to older adults with T2DM and overweight or obesity.
The effectiveness of hypocaloric diets (2,100 to 4,200 kJ/day deficit over 26 to 52 weeks) on weight loss in older adults with obesity (range, –2.3 to –10.7 kg) has been shown in a systematic review of randomised controlled trials (RCTs) [52]. However, both intentional and unintentional weight loss lead to muscle and bone loss [53,54], which exacerbates age-related declines in musculoskeletal health and physical function [55]. For this reason, the European Society for Clinical Nutrition and Metabolism (ESPEN) has recommended that for older adults with overweight (BMI ≥25 and <30 kg/m2), weight loss via hypocaloric diets should be avoided, and dietary strategies should be aimed at weight maintenance [56]. However, for older adults with obesity (BMI ≥30 kg/m2) and associated disorders (e.g., T2DM) weight loss via hypocaloric diets may be recommended depending on the risk-benefit profile for the person’s metabolic health, comorbidities, functioning, and quality of life [56].
Several dietary approaches can effectively induce weight loss for management of T2DM in adults. The recent Diabetes Remission Clinical Trial (DiRECT) using a ‘very low-calorie diet’ (VLCD; 3,500 kJ/day via total meal replacement for 3 months followed by structured food reintroduction) reported reductions in body weight (10.0 kg) and mean HbA1c (0.9%), with 46% of participants in the intervention group achieving T2DM remission at 12 months [57]. Despite their effectiveness, VLCDs are not recommended for older adults as they may lead to malnutrition and declines in muscle mass and physical function [56]. Accordingly, ESPEN guidelines advise that hypocaloric diets for older adults should not exceed a daily energy reduction of approximately 2,100 kJ/day and should maintain a minimum energy intake of 4,200 to 5,040 kJ/day. Importantly, diets should be well-balanced and ensure sufficient protein intake (at least 1 g/kg body weight/day) [56].
The Mediterranean diet may be a suitable weight loss dietary approach for older adults, as it is well-balanced and can be adapted to align with ESPEN’s recommended energy and protein requirements. The Mediterranean diet is characterised by high consumption of fruits, vegetables, legumes, and wholegrains; with olive oil as the main source of fat; and fish and poultry as the principal source of protein [58]. Meta-analyses of RCTs have suggested that the Mediterranean diet can induce weight loss and reduce HbA1c levels in adults with T2DM [58-60]. Further, emerging evidence suggests an association between greater adherence to the Mediterranean diet and protection against muscle mass loss and frailty [61,62].
Time-restricted eating (TRE) is a novel dietary approach that can achieve moderate energy intake restriction in older adults [57]. TRE can promote a small amount of weight loss in adults with overweight and obesity, by limiting eating, and therefore, energy intake, to a defined period of the 24-hour day (typically <12 hours) [63]. Qualitative studies have reported high satisfaction with TRE in adults with T2DM, noting it is relatively easy to follow as it does not require calorie counting nor changes to the types of food consumed [64,65]. A pilot study involving 10 older adults with overweight/obesity (mean age, 77.1 years; mean BMI, 34.1 kg/m2) reported high adherence (84%) to a 4-week TRE intervention with a self-selected 8-hour feeding window [66]. Mean weight loss over 4 weeks was 2.6 kg (no change in fasting glucose levels), with minor adverse events including decreased energy levels reported [66,67]. A major concern with prescribing TRE in individuals with T2DM is the risk of hypoglycemic events during fasting windows, especially in those taking sulfonylureas or exogenous insulin [68,69]. However, the few TRE trials to date that recruited participants taking sulfonylureas or insulin have reported no hypoglycemic events [70], and most reported reductions in body weight and improvements in glycemic indices [71-73]. TRE may therefore be safe and effective in individuals with T2DM, especially with frequent blood glucose monitoring, but additional clinical trials are needed including muscle mass as a primary endpoint.
The risks and benefits of weight loss need to be carefully evaluated in older adults with obesity and T2DM. If weight loss is warranted, prevention of malnutrition and preservation of muscle mass is integral when selecting a suitable dietary approach to reduce risk for sarcopenia. Supplementation of specific nutrients and performing exercise may therefore be important components of weight loss regimes for this population [56].
Protein, vitamin D, and omega 3 fatty acids
Proteins contain amino acids essential for muscle protein synthesis and there is some evidence to suggest increasing protein intake improves blood glucose management [74-77]. A recent meta-analysis of 12 RCTs (n=1,138) reported that consuming a high-protein diet had no beneficial effects on fasting plasma glucose, but it had a positive effect on homeostasis model assessment of insulin resistance (HOMA-IR) in individuals with T2DM [78]. Inconsistent findings in included RCTs could be related to factors such as differences in basal protein intake, types of protein consumed, or timing of intake. A subsequent meta-analysis of acute studies showed that whey protein supplementation decreased postprandial glycemia at 60 and 120 minutes compared with placebo [79]. This occurs due to the incorporation of protein into carbohydrate-containing meals delaying the release of glucose and may assist with managing blood glucose levels in this population [80]. Increasing protein intake to 1.2 to 1.6 g/kg of body weight promotes healthy aging, appetite regulation, weight management and helps maintain muscle mass and strength in older adults [81]. Protein supplementation supporting intakes exceeding approximately 1.2 g/kg of body weight may also enhance exercise-related improvements in muscle strength, mass and performance, particularly in those with low or inadequate basal intakes [82-85].
Vitamin D receptors are located within the pancreas and skeletal muscle and vitamin D deficiency is associated with poor metabolic and muscle health and physical performance [86-88]. However, these associations may be partly explained by confounding factors. For instance, obesity is common in T2DM, and it is a risk factor for vitamin D deficiency because a larger body size leads to greater volumetric dilution of vitamin D into tissues [89]. Low physical activity levels contribute to poor physical performance and sarcopenia, but they also lead to lower sunlight exposure, which is a risk factor for vitamin D deficiency. Nevertheless, there is some evidence from meta-analyses of RCTs showing that vitamin D supplementation improves glycemic indices in individuals with [90] and without T2DM [91], and also those with prediabetes [92,93], but not in populations with overweight or obesity [94]. With respect to improving muscle mass and function, older meta-analyses have reported beneficial effects, but only in individuals with 25-hydroxyvitamin D levels below 25 or 30 nmol/L [95,96]. Two recent meta-analyses showed no effect or adverse effects [97,98] on physical performance (short physical performance battery [SPPB] scores and timed up-and-go [TUG] times), which is supported by large vitamin D trials showing increased fall risk following high-dose supplementation [99,100]. Most studies identifying non-skeletal benefits following vitamin D supplementation reported them in populations with, or at risk of, moderate or severe vitamin D deficiency [90,95,96]. Thus, there appear to be very few benefits of vitamin D supplementation in populations with adequate vitamin D levels. Although there is some evidence that vitamin D holds promise as an adjunct therapy to enhance exercise-related improvements in physical function [101], recent RCTs have reported no benefits [102-104].
Long-chain omega-3 FA have anti-inflammatory benefits which may improve glycemic control and attenuate age-associated muscle loss, as both T2DM and sarcopenia are associated with chronic low-grade inflammation [105-108]. A meta-analysis of 45 RCTs showed that omega-3 FA supplementation led to significant improvements in lipid profile (decreased low-density lipoprotein and very low-density lipoprotein cholesterol and triglycerides) and reduced HbA1c, but changes in other glycemic indices such as fasting glucose, insulin levels and HOMA-IR were non-significant in individuals with T2DM [109]. Furthermore, the benefits on HbA1c became non-significant in a sensitivity analysis omitting two outliers showing very large treatment effects [109]. Other meta-analyses have shown no effect of omega-3 FA supplementation on glycemic indices [110,111]. The effects of omega-3 FA supplementation on muscle mass and function are also unclear. One of the largest meta-analyses conducted to date (66 studies) reported beneficial effects of omega-3 FA supplementation on some muscle mass and function outcomes (skeletal muscle mass and quadricep strength); however, other outcomes such as mid-arm muscle circumference and hand grip strength were unchanged [112]. Current evidence does not support the provision of omega-3 FA supplementation for managing T2DM or sarcopenia.
Given that older adults are at high risk of malnutrition and some individuals struggle with maintaining oral intake of food to sustain diet quality, multi-supplement interventions are promising in this population [113-115]. Multi-supplements may be beneficial if doses are not reduced for convenience (e.g., taking a single multi-supplement with several nutrients at low doses, as opposed to taking individual nutrients at recommended/higher doses). However, multi-supplement interventions are unlikely to be beneficial in those who have an adequate dietary intake from well-balanced diets.
Physical activity
Physical activity, defined as any bodily movement produced by skeletal muscles that results in energy expenditure [116], plays a significant role in the prevention and management of T2DM and sarcopenia. The World Health Organization (WHO) recommends that older adults with chronic conditions accumulate 150 to 300 minutes per week of moderate to vigorous intensity physical activity (MVPA) and limit sedentary time [117]. Canada has adopted 24-hour movement guidelines for older adults which include similar MVPA targets and additional guidance to limit sedentary time to 8 hours or less, and to break up periods of sitting as often as possible [118]. However, large observational studies using objective measures indicate approximately 80% to 95% of older adults do not achieve the minimum target for MVPA [119], and can spend up to 65% to 80% of their waking day sedentary [120]. Given low physical activity and high sedentary behavior are prospectively associated with worse health outcomes in older adults including development of T2DM [121], cardiovascular disease [122], lower muscle strength and power [123], and all-cause mortality [124], strategies to get older adults moving more and sitting less are of paramount importance.
Regular physical activity is recommended for management of glucose control in older adults with T2DM [125,126]. However, meta-analyses indicate that interventions aimed at changing people’s behavior to increase physical activity with limited focus on other interventions, such as consuming a hypocaloric diet, generally do not improve glycemic markers [127,128]. One meta-analysis demonstrated that physical activity interventions were more successful at improving glycemic control if they included structured/supervised exercise as opposed to behavioral intervention alone [127], and exercise will be discussed later in this review.
In recognition of the need to prevent frailty, falls, and fragility fractures in older adults, the WHO also recommends that: “As part of their weekly physical activity, older adults should do varied multicomponent physical activity that emphasizes functional balance and strength training at moderate or greater intensity, on 3 or more days a week, to enhance functional capacity and to prevent falls” [116]. A recent meta-analysis in older adults (112 studies; n=43,796) with and without chronic diseases showed that higher total physical activity levels and MVPA were associated with better hand grip strength and chair stand times [123]. Associations were also stronger between higher physical activity levels and higher lower-limb muscle strength and power measures, compared with upper-limb measures [123]. Physical activity interventions have both short- and long-term benefits for physical performance. In the Look Action for Health in Diabetes (AHEAD) trial, adults with T2DM and overweight or obesity (aged 45 to 76 at enrolment; BMI ≥25 kg/m2) were randomised to either intensive lifestyle intervention (ILI; individual weight loss goal of ≥10% and physical activity goal of ≥50 minutes per week in the first month and ≥175 minutes per week by the end of 6 months) or diabetes support and education (DSE; control) [129]. Between years 1–8, self-reported physical function remained significantly higher in the ILI group compared with DSE [130]. At 8-year post-randomisation, the ILI group had superior SPPB scores and gait speed compared with DSE [131]. At approximately 11 years post-randomisation and 1.5 years after the intervention ended, the ILI group still had 26% lower odds of having slow gait speed (<0.8 m/sec) compared with DSE [132]. Participants in the ILI group had considerable support from a lifestyle coach throughout the study, which likely contributed to long-term physical performance benefits.
Sedentary behavior
Interventions aimed at increasing MVPA alone can inadvertently lead to behavioral compensation whereby light-intensity activity is decreased and sedentary behavior is increased [133-135]. It is possible that metabolic and muscle health benefits of physical activity interventions can be blunted or eliminated by compensatory increases in sedentary behavior. High levels of MVPA (60 to 75 minutes per day) are required to offset the deleterious effect of sitting for >8 hours per day on all-cause mortality [136]. However, these levels of MVPA still may not be sufficient to offset the effects of sedentary behavior on metabolic health in older adults. A recent study of 54 extremely active older adults (mean age, 71 years) demonstrated that high volumes of sedentary behavior (9.4 hours/day) adversely influences metabolic health, even in the presence of high volumes of MVPA (2.6 hours/day) [136]. This suggests interventions should target both increasing MVPA and reducing sedentary behavior for optimal improvement in metabolic health in older adults.
Several short-term studies have investigated the acute metabolic effects of intermittent breaks in prolonged sitting in older adults (mean age ≥60 years) with T2DM [137-141]. These studies have reported reduced postprandial glucose and/or insulin levels [137,140,141], improved 22 to 24 hours continuous glucose monitoring profiles [138-140,142], and improved vascular function [143]. It is thought that intermittent activity improves glycemic control partly because it can be spread across multiple postprandial periods. Some of these studies even compared intermittent physical activity spread across the day to an energy-matched discrete bout of exercise demonstrating comparable [139], or superior glucose-lowering effects with intermittent physical activity [138,144]. This suggests that an effective intervention for improving glucose control in older adults with T2DM is to avoid prolonged periods of sitting and to spread physical activity across the day, particularly after meals. However, while there is evidence of small improvements in glycemic indices in some long-term studies [145-147], further RCTs are needed to confirm this.
Evidence from longitudinal observational studies with objective measures suggests that reducing sedentary behavior is associated with improved physical function and reduced falls risk in older adults [123,148]. However, other longitudinal observational studies in older adults have shown that higher amounts of MVPA are associated with a decreased likelihood of sarcopenia and/or its components (low appendicular lean mass and grip strength and slow TUG time), regardless of the amount of sedentary behavior [149,150]. Evidence from interventions specifically targeting sedentary behavior is inconclusive [151-153]. Some demonstrate improved physical function [151-153], even in the absence of observed reductions in sitting time [153], while others report reductions in sedentary time do not affect physical function [154]. Nevertheless, interventions specifically targeting reductions in sedentary behavior may be more acceptable to some older adults than interventions that increase MVPA [155].
Taken together, evidence suggests both increasing MVPA and reducing sedentary behavior improves metabolic and muscle health in older adults. Additional high-quality evidence from large RCTs is needed to better inform physical activity guidelines for those with T2DM and sarcopenia.
Exercise
Exercise is a planned, structured, and repetitive subset of physical activity with the objective to improve or maintain physical fitness [116]. Exercise interventions have well-established benefits for multiple health outcomes, including metabolic and musculoskeletal health markers such as insulin sensitivity [156,157], glycemic control [158], skeletal muscle mass [159], strength [160,161], and bone mass [162].
The benefits of exercise interventions may be influenced by various factors, including the mode/type of exercise performed (e.g., aerobic vs. resistance exercise) and prescription-related factors such as frequency, intensity, and duration of exercise sessions. Aerobic exercise is a form of repetitive/rhythmic structured physical activity that uses large muscle groups and can be maintained continuously, it includes activities such as jogging, cycling and swimming. Resistance or muscle-strengthening exercise involves repetitive muscular contractions against ex-ternal resistance, such as lifting weights or body weight alone. Short- and long-term physiological adaptations to aerobic and resistance exercise are somewhat distinct, with aerobic exercise primarily associated with improvements in cardiometabolic health markers (such as cardiorespiratory fitness, insulin sensitivity, and glycemic control), and resistance exercise mainly targeting aspects of musculoskeletal health (such as skeletal muscle mass, strength, and bone mineral density). There is, however, some cross-over in the specificity of responses and adaptations to aerobic and resistance exercise, particularly following shorter-term interventions and in those with lower baseline fitness levels [158,163,164]. Understanding the role of exercise prescription-related factors in addressing consequences of T2DM and/or sarcopenia can inform practical guidelines for management of these diseases.
Several RCTs have investigated whether exercise interventions (up to 96 weeks), involving either aerobic or resistance training alone, or in combination, can improve markers of metabolic health in older adults with T2DM [165-170]. Overall, these studies provide evidence that in those with T2DM, both supervised aerobic and resistance training are effective for improving HbA1c and fasting blood glucose levels [171]. In a RCT of 100 adults aged ≥55 years with T2DM, the effects of three different 16-week exercise interventions (aerobic exercise, resistance exercise, or a combination of both [same intensity but half the volume of other exercise groups]) on various metabolic health markers were compared with a control group encouraged to perform low-to-moderate intensity aerobic activities (e.g., brisk walking, cycling) for 150 minutes per week [165]. At follow-up, all exercise conditions showed improved metabolic health markers from baseline, including reduced fasting plasma glucose, HbA1c, fasting plasma insulin, and HOMA-IR, with no differences between conditions (including vs. the control condition, which showed no within-group changes in these outcomes). These improvements occurred independent of weight loss. Although both aerobic and resistance training appear to independently improve glycemic control, a network meta-analysis showed that the combination of both is more effective for reducing HbA1c than either mode alone in individuals with T2DM [172].
The benefits of resistance training for improving muscle mass and function in older adults with sarcopenia are well-established and it is a first-line treatment for this condition [173,174]. A recent systematic review and meta-analysis of 26 studies in 1,191 older individuals with sarcopenia (age range, 60.6 to 89.5years) [173] found resistance training was effective for improving knee extensor strength and measures of physical function (gait speed, TUG test, but not chair stand test performance). Resistance training prescription factors, such as frequency, intensity, and dose, may influence the effectiveness of such interventions. Indeed, a meta-analysis of 15 studies found that in older adults (mean age, 67.8 years), improvements in muscle strength and size following resistance training tended to be greater when involving higher loads (approximately 80% one-repetition maximum [1-RM]) compared with lower loads (approximately 45% 1-RM) [175]. However, these effects were substantially smaller in studies that work-matched both higher- and lower-load resistance training protocols [175].
Pragmatic and time-efficient approaches including “exercise snacking” may be effective for improving markers of glycemic control [176]. “Exercise snacks” may be considered as isolated bouts of exercise lasting ≤1 minute and performed multiple times throughout the day [177]. Various studies have shown the efficacy of exercise snacking approaches [176,178-180] for interrupting short-term sedentary behavior patterns and improving cardiometabolic health markers in preclinical and clinical populations. While some studies have found shortterm benefits of exercise snacking for improving indices of metabolic health and glycemic control [176,178-180], there is less evidence for the long-term efficacy of this approach.
A few studies have also explored the effects of exercise snacking on musculoskeletal health in older adults [181-183]. A pilot study in older adults (age range, 65 to 80 years) showed that twice-daily “exercise snacks” (five bodyweight exercises performed for 1 minute with 1-minute passive recovery between exercises; 9 minutes total session duration) performed for 28 consecutive days improved 60-second sit-to-stand performance, and large effect sizes were noted for the difference in the change in leg press power and thigh muscle cross-sectional area versus control [182]. Another pilot study [181] using a similar resistance-based “exercise snacking” protocol in older adults (mean age, 70 years) noted positive findings regarding the feasibility and acceptability of this exercise approach, but there was little evidence of physical function benefits versus control (no exercise; study was not powered to detect differences in physical function) when assessed remotely after 4 weeks. Overall, while the limited available evidence has shown mixed benefits of exercise snacking approaches for improving measures of metabolic and musculoskeletal health, the positive findings regarding the feasibility and acceptability of these approaches highlight the need for further work to determine and improve their efficacy in older adults, particularly those with sarcopenia and/or T2DM [181-184].
Combined exercise and hypocaloric diet interventions
As described earlier, weight loss may lead to muscle mass losses, but is beneficial for metabolic health in older adults with T2DM, and exercise can augment weight loss-related metabolic health improvements [169]. In sedentary older adults (aged 60 to 80 years) with overweight and T2DM consuming a hypocaloric diet, performing supervised and structured high-intensity resistance training (nine exercises; eight to 10 repetitions; 75% to 85% 1-RM) further reduced HbA1c levels after both 3 and 6 months (–0.5% and –0.8%, respectively) [169]. In 160 older adults with obesity consuming a hypocaloric diet, completing concurrent combined aerobic and resistance training led to an 86% improvement in insulin sensitivity index, which was significantly better than completing either exercise mode alone [185].
Exercise also influences changes in muscle mass, IMAT and physical function that occur following hypocaloric diet interventions. In 107 older adults with obesity consuming a hypocaloric diet for 12 months, combined resistance and aerobic exercise led to greater improvements in a composite physical performance score compared with no exercise (21% vs. 12%, P<0.001) [186]. Another study in older adults with obesity (n=160) comparing different exercise modes during a hypocaloric diet intervention showed that combined resistance and aerobic exercise led to greater improvements in a composite physical function score (21%) compared with aerobic (14%) and resistance exercise (14%), although this may be attributed to the higher training dose in this group [187]. Interestingly, those completing aerobic exercise had significantly greater lean mass losses (–5%) than the resistance exercise (–2%) and combined resistance and aerobic exercise (–3%) groups [187]. In support of the abovementioned findings, a recent meta-analysis showed that resistance exercise attenuates muscle mass losses that occur following weight loss [53]. Only one RCT has compared changes in IMAT between older adults with obesity undertaking a hypocaloric diet intervention alone, or in combination with resistance or aerobic exercise for 18 months [188]. The greatest decreases in trunk and thigh IMAT area were observed in the group performing concurrent aerobic training, followed by the resistance training group, and then the no exercise training group (between-group differences were significant but post-hoc tests were non-significant). All groups had significant improvements in trunk and thigh IMAT area relative to baseline [188]. More studies are required to determine optimal exercise prescriptions for improving metabolic and muscle health in older adults with T2DM and/or sarcopenic obesity consuming a hypocaloric diet.
Medications
T2DM is managed using several types of medications (e.g., biguanides, dipeptidyl peptidase inhibitors, sodium-glucose cotransporter-2 inhibitors, sulfonylureas, thiazolidinediones, insulin) and their mixed effects on muscle health and function have been described previously [6,189]. However, use of new generation incretin-based medications has increased recently due to their ability to treat both poor glycemic control and obesity. Glucagon-like peptide-1 (GLP-1) receptor agonists are the most common incretin-based medications, which lower glucose levels by stimulating insulin secretion from pancreatic β-cells [190]. They also suppress appetite, delay gastric emptying, improve satiety, and decrease glucagon secretion [190]. In a recent RCT of 1961 adults with overweight and obesity, once-weekly subcutaneous semaglutide (2.4 mg) led to a 15% decrease in body weight over 68 weeks compared with a 2.4% reduction in placebo (both groups also underwent a lifestyle intervention involving 500 kcal/day energy restriction and increased physical activity [150 minutes per week] was encouraged) [191]. Approximately 86% of participants had clinically significant weight loss (≥5%) in the semaglutide group versus 32% in placebo and these participants also had improvements in fasting glucose, HbA1c, and self-reported physical function [191]. While a significant decrease in absolute lean (muscle) mass was observed, percentage lean mass increased, relative to placebo [191]. Another incretin-based medication, tirzepatide, is a dual glucose-dependent insulinotropic peptide and GLP-1 agonist. Tirzepatide leads to similar improvements in the abovementioned outcomes in individuals with overweight and obesity over a similar timeframe [192]. In a recent head-to-head study, tirzepatide led to greater reductions in body mass and HbA1c compared with semaglutide in individuals with T2DM, although the semaglutide dose was 1 mg (once-weekly) [193]. Dual-agonists appear to confer additional benefits over mono-agonists, and other dual- and tri-agonists currently in clinical trials show great promise (NCT04478708 [194,195]). Future studies on these medications would benefit from comprehensively measuring physical function outcomes and prescribing targeted exercise to attenuate weight loss-related declines in muscle mass.
Currently there are no approved pharmacological treatments for sarcopenia; however, several medications have shown potential efficacy in recent clinical trials. Myostatin-based medications are of great interest given the inhibition of this myokine significantly increases muscle mass in animal knockout/loss-of-function models [196,197]. Myostatin propeptides, soluble receptors, antibodies, and endogenous antagonists have been developed and some have undergone testing in clinical trials [198-200]; myostatin-based treatments consistently increase muscle mass, but they have limited or no benefits for physical function, which is a more clinically relevant outcome for patients [198-200]. Recent trials demonstrated that bimagrumab can decrease fat mass and improve insulin sensitivity and HbA1c in individuals with insulin resistance and T2DM [201,202], but larger trials are required to establish the safety and efficacy of this treatment. Testosterone treatment is effective for increasing muscle mass and to a lesser degree, muscle strength, but similar to myostatin-based agonists there is inconsistent evidence supporting beneficial effects on physical performance in older populations with and without T2DM [203,204]. There are no clinical trials assessing efficacy of testosterone for improving muscle health in older adults with diagnosed sarcopenia. Testosterone also decreases risk of incident T2DM in middle-aged and older men [205], with glycemic improvements being mediated to the greatest degree by treatment-related changes in fat mass [206]. Despite these positive findings, there is still insufficient evidence to support routine use of this treatment in older adults with T2DM without pathological hypogonadism [205,207]. To overcome adverse androgenic/virilising side effects associated with testosterone [208,209], selective androgen receptor modulators (SARMs) have been developed to stimulate anabolic effects in skeletal muscle and other tissues (e.g., bone) while minimising adverse effects on other tissues (e.g., prostate) [210,211]. Only one study has explored the effects of a SARM in a population with sarcopenia [212]. In 170 postmenopausal women (aged >65 years), a SARM (MK-0773) taken for 6 months led to a 0.6 kg increase in appendicular lean mass relative to placebo, but it did not lead to any improvements in muscle strength or performance [212]. SARMs have shown some promise in other populations with respect to increasing muscle mass, but effects on physical function are unclear [213,214]. Increasing muscle mass via pharmacological treatments provides limited functional benefits, so the addition of targeted exercise (particularly resistance training), will likely be key in achieving physical function-related endpoints in future clinical trials.
Endoscopic bariatric therapies
Bariatric surgery (BS) is the most effective intervention for long-term weight loss for individuals with obesity and its comorbidities [215]. BS-induced weight loss improves glycemic control with T2DM remission rates ranging from 69% to 90% depending on the type of surgery [216,217]. Achievement of T2DM remission following BS is multi-factorial but largely driven by weight loss-related improvements in insulin sensitivity and β-cell function, and there are also documented changes in gut physiology, bile acid metabolism, and gut microbiota that appear to have benefits on glycemia [218]. Although the beneficial effects of BS on metabolic health are well-established, its effect on muscle health is unclear.
A prospective cohort study in 47 adults (mean age, 42 years) who underwent Roux-en-Y gastric bypass (RYGB) showed that total lean mass decreased by 13% and absolute hand grip strength decreased by 9%, however, relative hand grip strength (normalized to BMI) increased by 32% [219]. The same study reported an increase in gait speed (0.1 m/s) and improved 400 m walk time [219]. A recent meta-analysis showed that RYGB results in greater 12-month body weight and fat loss, but similar losses in lean mass, compared with adjustable gastric band, although this was based on only two RCTs [220]. Non-surgical intragastric balloons, involving endoscopic insertion of a silicon fluid-filled balloon into the stomach causing earlier feelings of satiety [221], also lead to lean mass losses [222], despite improving glycemic control [223]. Maintaining muscle mass should therefore be a key focus following BS.
Exercise augments BS-related metabolic, body composition and physical function improvements. A recent RCT showed that 62 adults with obesity (age range, 18 to 55 years) randomised to combined resistance and aerobic training following RYGB had greater improvements in insulin sensitivity (Matsuda index, 325%) than those randomised to standard care following RYGB [224]. A meta-analysis showed that exercise training (aerobic and/or resistance exercise and/or high-intensity interval training) led to greater total body mass (mean difference [MD], –1.8 kg) and fat mass (MD, –2.1 kg) losses, but similar lean mass losses (MD, 0.7 kg), compared with controls receiving standard care, following BS [225]. Exercise also improved muscle strength (based on different strength tests) and endurance (based on different walking tests) [225]. Although performing any type of exercise following BS is likely to be beneficial, performing resistance training is particularly important for attenuating losses in muscle mass and function, especially in older patients.
EMERGING APPROACHES TO FACILITATE MANAGEMENT OF T2DM AND SARCOPENIA
Digital health technologies
The WHO describes digital health as “the field of knowledge and practice associated with the development and use of digital technologies to improve health.” Digital health technologies are an effective and valued tool for delivering and monitoring remote diet and exercise interventions targeting glycemic control in older adults with and without T2DM [226,227]. These interventions use delivery approaches such as video-conferencing, phone calls, text messages, smartphone and tablet-based applications and other digital inputs [228], and improve practitioner-patient communication and support, as well as access to medical services [229]. The Weight Achievement and Intensive Treatment (Why WAIT) 12-week multidisciplinary weight management program was delivered via a smartphone application and video-conferencing during the coronavirus disease 2019 (COVID-19) pandemic [230]. This intervention for individuals with obesity (BMI, 30 to 45 kg/m2) and diabetes (type 1 or 2) [231] involved a hypocaloric diet and exercise, as well as a cognitive-behavioral intervention and group education. The 16 participants who completed the digital health program had similar changes in body weight (–6.8 kg vs. –7.4 kg), HbA1c (–1.0% vs. –1.03%) and other metabolic outcomes compared with 22 participants who completed the in-person program before the pandemic [230]. Several other studies involving web-based dietary interventions have also reported improvements in HbA1c after 4 months (–0.30%) [232], 12 months (–0.24%) [233], and 5 years (–0.29%) [234] in adults and older adults with T2DM. A recent systematic review found that 20 out of 21 studies that delivered digital interventions for the management and prevention of T2DM showed significant within-group improvements in at least one key T2DM-related outcome (e.g., HbA1c, blood glucose levels, weight loss) and 85% (11/13) of parallel-group studies showed a significant improvement compared with control [235].
Only a few studies have explored effects of digital health interventions on muscle mass and/or function in older adults. A recent 12-month RCT explored the feasibility, acceptability and efficacy of the successful Lifestyle integrated Functional Exercise (LiFE) intervention (involving the incorporation of strength and balance exercises into daily activities) [236] in older adults aged between 61 and 70 years, delivered via smartphone and smartwatch [237]. The program appeared to be feasible with 58 out of 61 participants in the virtual group using the application for an average of 180 days, but had no benefits on hand grip strength or gait speed [237]. Another 12-week RCT assessed the effects of a home-based exercise (resistance band-based exercises, 10 repetitions and five sets, performed 3 days per week) and hypocaloric dietary intervention (1,200 kcal/day) delivered via digital communication applications (e.g., LINE, FaceTime) on muscle mass and function, in older adults (≥55 years) with overweight or obesity (BMI range, 27 to 35 kg/m2) and knee osteoarthritis [238]. Participants were randomised into three groups; diet alone, exercise alone, and combined exercise and diet. Lower-limb muscle mass increased in the exercise alone group and decreased in the other two groups (losses were attenuated in combined exercise and diet) [238]. TUG times improved in all groups and significantly more in the combined exercise and diet group compared with exercise alone [238]. It is currently unclear how digital health interventions affect muscle health and function in older adults with T2DM and/or sarcopenia.
Collectively, the above studies demonstrate that digital health interventions can have similar efficacy compared to in-person interventions; however, digital health technologies can be challenging for patients, and may require resource-intensive training and support by a health professional [234,239]. A recent scoping review evaluated barriers to digital health in 14 qualitative studies of older adults (≥60 years) and identified that small screens and text size, reduction of fine motor control and cognitive concerns related to memory, remembering passwords and logging in were commonly cited concerns [240]. However, older adults can overcome barriers to engaging with digital technology. An observational study (n=2,169) analysing data from a fully automated digital health platform reported that older adults had significantly more digital coaching conversations, logged more meals, and recorded more device measurements than younger users (35 to 64 years) over 12 months [241]. Further research is required to determine the efficacy and feasibility of multicomponent digital health interventions, using different technologies, and how these can enhance older adults’ self-management of sarcopenia and/or T2DM.
Health literacy
T2DM and sarcopenia are both chronic diseases where self-management is the primary means of treatment. Individuals often need to make major lifestyle changes to adhere to dietary and physical activity recommendations and follow new medication regimens. These behavior changes can be difficult to initiate and maintain [242], and individuals require health literacy (HL) skills to self-manage their health [243]. HL is a multidimensional concept defined as ‘the personal characteristics needed for an individual to access, understand, appraise and use information about health and health care services to make decisions about health’ [244]. The risk of developing T2DM is increased in individuals with low or inadequate HL [245,246]. A meta-analysis of 29 observational studies (n=13,457 participants) reported that almost 33% of patients with T2DM have low HL [247]. This is problematic because higher HL is associated with greater T2DM health knowledge and glucose control [248,249] and increased uptake of positive self-management behaviors such as medication adherence, physical activity and healthy diet [250-253].
In a recent systematic review of 14 RCTs [254], HL-focused interventions led to significant improvements in T2DM health knowledge, quality of life, physical activity levels, and health-related self-efficacy, although inconsistent results were seen across self-management strategies such as glycemic control, foot care, diet management, and medication adherence [254]. However, this review was highly education-focused and included studies that interchangeably used the terms ‘health literacy’ with ‘health education.’ While education often plays an important role in HL interventions, providing education alone is not considered a ‘HL approach’ and is rarely sufficient to facilitate behavior change to sustain optimal health outcomes [255].
The ‘teach-back method’ can be considered a HL intervention because it aims to improve patient-provider interactions by repeatedly checking and clarifying health information given to patients until the patient can correctly recall the information given [256]. Teach-back is effective across a wide range of settings, populations, and outcome measures, including patients with T2DM [256]. Clinical trials have reported significant improvements in medication adherence, diet changes, and foot self-care in populations with T2DM after implementation of the ‘teach-back method’ compared with usual care control groups [257-259]. More recent RCTs assessing HL-based counselling and/or behavior change interventions have shown positive results for patient activation (an individual’s knowledge, skills, and confidence for managing their health) [260], diabetes self-management behaviors [260,261], and self-efficacy [261,262]. The evidence supporting the effectiveness of HL-oriented interventions on physical activity and exercise in patients with T2DM is limited, although a systematic review of six interventional studies (n=980) reported generally positive findings across several physical activity behaviors in individuals with T2DM [263].
Two RCTs of a HL-oriented behavior change program for exercise, diet and nutrition reported significant improvements in components of sarcopenia (TUG test; gait speed; grip strength), as well as improvements in physical activity levels and dietary variety scores for older adults compared to a standard control group [264,265]. No studies have investigated associations between HL and sarcopenia, although, one longitudinal study [266] found that older adults with lower socioeconomic factors (education, occupation), which influence HL levels, were more likely to have lower grip strength and lean mass. Another cross-sectional study in the same cohort also reported that lower educational attainment, but not occupation, was associated with increased likelihood for both obesity and sarcopenic obesity in community-dwelling older adults [267]. There is a need for further research regarding the effectiveness of HL interventions, and whether they can enhance other treatments and therapies, in older adults with metabolic and musculoskeletal diseases [268].
CONCLUSIONS
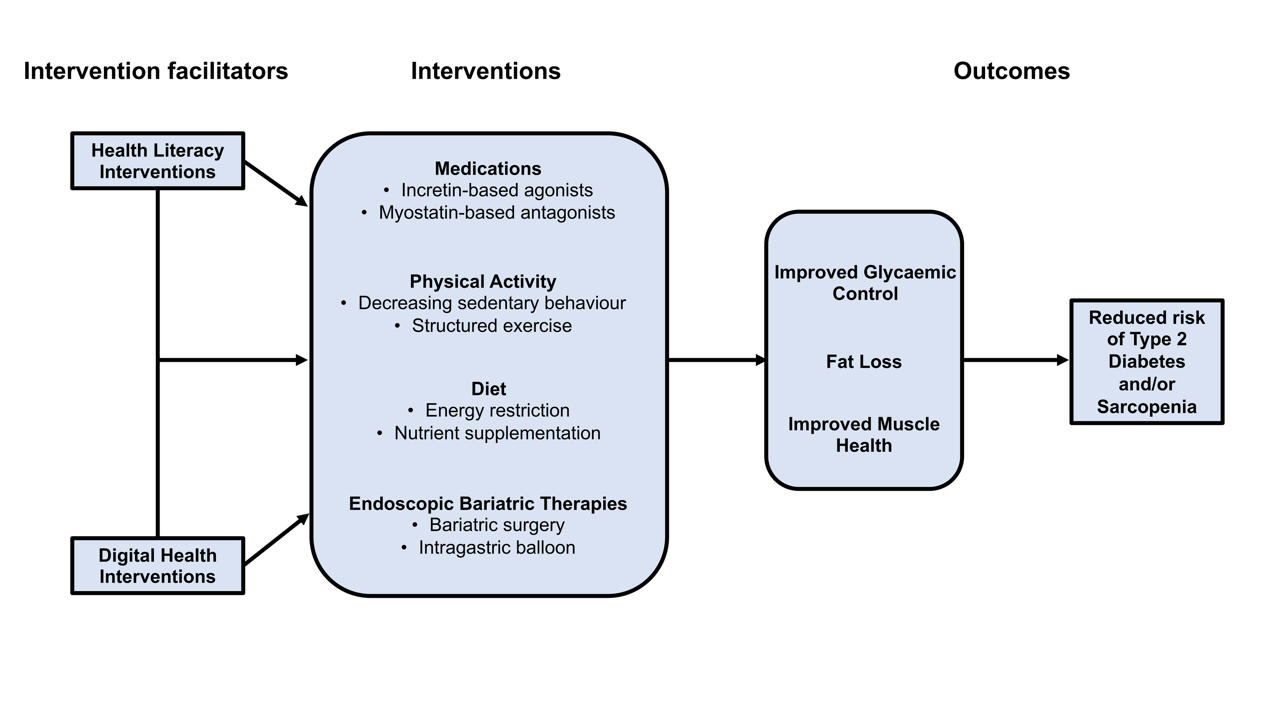
There is considerable cross-sectional data highlighting the bidirectional relationship between T2DM and sarcopenia, but longitudinal data are lacking. Myosteatosis is likely a significant contributor to this relationship via direct effects on insulin resistance and physical function. Increasing physical activity and decreasing sedentary behavior have independent benefits on metabolic and muscle health, and may be achieved in by breaking up prolonged sedentary periods with brief activity or exercise bouts (e.g., activity breaks or ‘exercise snacks’). Nevertheless, structured exercise has the most pronounced effect on these aspects of health, and different modalities have distinct but overlapping benefits, so multi-modal interventions should be prioritised with a key focus on resistance training in this population. Weight loss is the most effective method for improving metabolic health and can be achieved via hypocaloric diets, using pharmacotherapies and/or undergoing surgical or non-surgical endoscopic bariatric procedures. However, in the absence of exercise, weight loss can have detrimental effects on muscle mass, potentially increasing risk for sarcopenia. As described in Fig. 2, uptake of, and adherence to, the abovementioned treatments and therapies for improving metabolic and muscle health may be facilitated or enhanced via the use of digital health interventions or targeting individual or system-level HL barriers.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None
Acknowledgements
None