The Risk of Shoulder Adhesive Capsulitis in Individuals with Prediabetes and Type 2 Diabetes Mellitus: A Longitudinal Nationwide Population-Based Study
Article information
Abstract
Background
This study aimed to investigate the association between type 2 diabetes mellitus (T2DM) and shoulder adhesive capsulitis (AC) using a large-scale, nationwide, population-based cohort in the Republic of Korea.
Methods
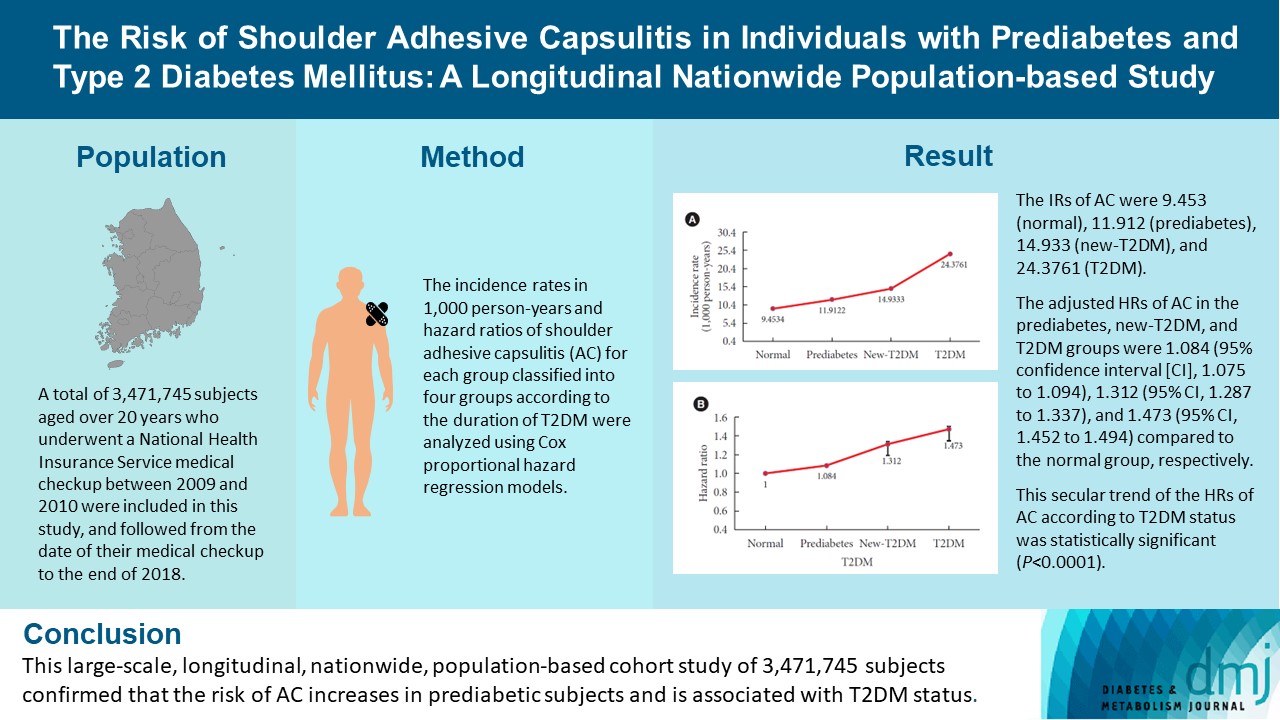
A total of 3,471,745 subjects aged over 20 years who underwent a National Health Insurance Service medical checkup between 2009 and 2010 were included in this study, and followed from the date of their medical checkup to the end of 2018. Subjects were classified into the following four groups based on the presence of dysglycemia and history of diabetes medication: normal, prediabetes, newly diagnosed T2DM (new-T2DM), and T2DM (claim history for antidiabetic medication). The endpoint was new-onset AC during follow-up. The incidence rates (IRs) in 1,000 person-years and hazard ratios (HRs) of AC for each group were analyzed using Cox proportional hazard regression models.
Results
The IRs of AC were 9.453 (normal), 11.912 (prediabetes), 14.933 (new-T2DM), and 24.3761 (T2DM). The adjusted HRs of AC in the prediabetes, new-T2DM, and T2DM groups were 1.084 (95% confidence interval [CI], 1.075 to 1.094), 1.312 (95% CI, 1.287 to 1.337), and 1.473 (95% CI, 1.452 to 1.494) compared to the normal group, respectively. This secular trend of the HRs of AC according to T2DM status was statistically significant (P<0.0001).
Conclusion
This large-scale, longitudinal, nationwide, population-based cohort study of 3,471,745 subjects confirmed that the risk of AC increases in prediabetic subjects and is associated with T2DM status.
INTRODUCTION
Shoulder adhesive capsulitis (AC) is a common disease characterized by a progressive and painful loss of passive and active shoulder range of motion. The prevalence of AC is between 2% and 5% in the general population and the majority of patients are female [1]. AC is a painful shoulder disease in which chronic inflammation of the capsule causes capsular thickening, fibrosis, and adhesion of the capsule to the surrounding soft tissue and anatomic neck of the humerus [2].
Systemic conditions such as diabetes mellitus (DM), obesity, dyslipidemia, thyroid disease, cardiac disease, Dupuytren contracture, breast cancer treatment, and neurologic disorders increase the risk of AC [1-9]. Diabetic patients have a 2 to 4-fold higher risk of developing AC compared to the general population [10]. A nationwide, population-based cohort study [9] performed in Taiwan revealed that the incidence of AC in a DM cohort was 3.08 times that of the comparison cohort (146.9 vs. 47.7 per 10,000 person-years). Also in that study, the hazard ratios (HRs) of AC for diabetic patients remained significantly higher than those for non-diabetic patients (P<0.001). Several studies have demonstrated that the hyperglycemia associated with DM can trigger collagen matrix changes within the joints [11,12]. These changes ultimately induce the fibrotic and inflammatory alterations associated with the histochemical status of the disease [3,13]. In addition, Esposito et al. [5] reported that hyperglycemia itself is a pro-inflammatory state.
The prevalence of DM in adults has increased worldwide over the past few decades [14]. The International Diabetes Federation (IDF) warns that the global number of diabetic patients will increase to 642 million by 2040, with an overall prevalence of 10.4% in the worldwide population [15]. DM can damage all organs of the body, including the musculoskeletal system, and can cause many health complications [16]. Thus, the increasing prevalence of DM could lead to a larger public health burden.
Considering the increasing prevalence of DM worldwide, this study aimed to analyze the relationship between the duration of type 2 diabetes mellitus (T2DM) and AC through a large-scale, nationwide, population-based cohort study conducted in the Republic of Korea. To the best of our knowledge, this study is the first nationwide population-based cohort study to demonstrate an association between T2DM (including prediabetes) and AC. We investigated the HRs and incidence rates (IRs) of AC in subjects with prediabetes and T2DM. Subjects with T2DM were subdivided into two groups according to their history of T2DM medication use to evaluate the influence of T2DM on the IRs and HRs of AC. We confirmed that the risk of AC differs by DM status, and compared the risk of AC between T2DM and non-T2DM populations.
METHODS
Data collection
This study used data from the Korean National Health Insurance Service (NHIS) Claims Database (in which diagnoses are recorded using International Classification of Diseases, Tenth Revision [ICD-10] codes), which contains all yearly claims data for the Korean NHIS program, the Korean Medical Aid program, and long-term care insurance. In the Republic of Korea, all citizens are mandatorily enrolled in the NHIS; thus, the system provides coverage for almost the entire Korean population. Therefore, the Korean NHIS database is considered to represent the entire Korean population and has been used in population-based studies.
NHIS enrollees underwent a medical examination including measurements of height, weight, blood pressure, and waist circumference, as well as laboratory tests such as fasting glucose, cholesterol, triglycerides, and serum creatinine. Data on past medical history and health-related behaviors such as smoking, alcohol intake, and physical activity were collected using standardized self-report questionnaires. Quality control procedures for laboratory tests were performed in accordance with the Korean Association of Laboratory Quality Control. This study was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of our institution (IRB No. SSU-202007-HR-236-01). Informed consent was waived by the board.
Study population
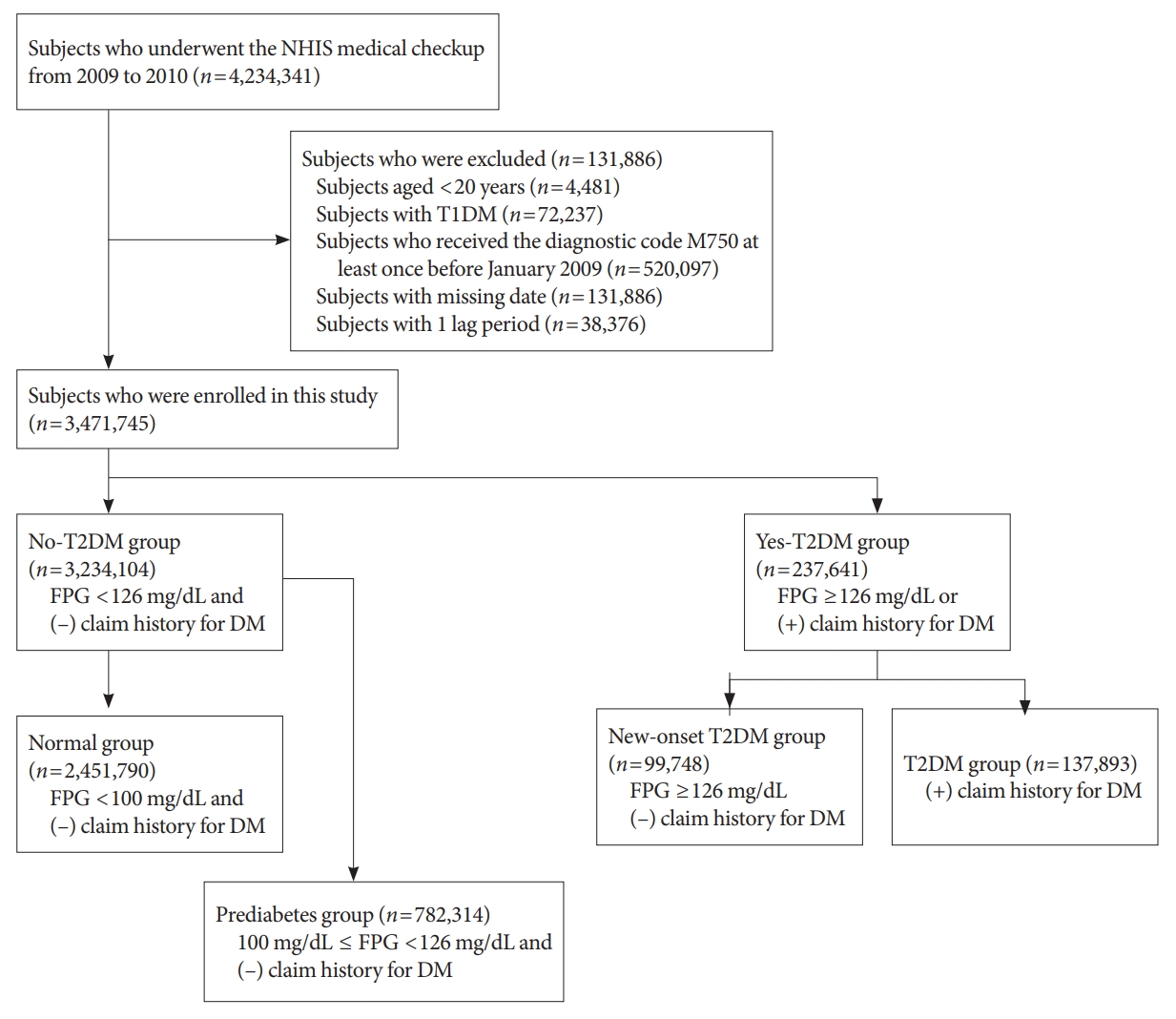
In this longitudinal, nationwide, population-based cohort study, all subjects who underwent an NHIS medical checkup between January 1, 2009 and December 31, 2009, were extracted from the medical checkup database. The date of the checkup was used as the baseline. The endpoint was new-onset AC during follow-up, which corresponded to when patients had visited the hospital or private clinic three times within 1 year and received a diagnostic code of AC (ICD-10 code, M750). Regular short-term follow-up is typical to assess the recovery of shoulder joint range of motion in the early phase of AC treatment. We excluded patients with ICD-10 code M750 who visited the hospital only once or twice, to reduce selection bias. We also excluded patients younger than 20 years (n=4,481), those with type 1 diabetes mellitus (T1DM) (n=72,237), those who had ever visited a hospital and received diagnostic code M750 at least once before January 2009 (n=520,097), and those with missing data (n=131,886). In addition, to prevent reverse causality, we excluded patients who had ever received diagnostic code M750 or died within 1 year of the date of medical evaluation provided by the Korean National Health Insurance Corporation (defined as 1 lag period; n=38,376). Finally, a total of 3,471,745 subjects were enrolled in the study (Fig. 1). The study population was followed from baseline (date of NHIS medical checkup) until the endpoint (new-onset AC) or December 31, 2018 (whichever was first).
Definition of diabetes
DM status was determined based on laboratory test results from the NHIS medical checkup, and from the claims history for antidiabetic medications under ICD-10 code E11–14 before the checkup (from January 1, 2002, to the baseline). A fasting plasma glucose (FPG) level ≥126 mg/dL was defined as T2DM. The study population was initially divided into no-T2DM and yes-T2DM groups (Fig. 1). Subjects who had a FPG level <126 mg/dL and no claims history for antidiabetic medication were classified into the no-T2DM group, which was further divided into normal and prediabetes groups. The normal group included subjects with a FPG level <100 mg/dL. The prediabetes group included subjects with a FPG level of 100 to 126 mg/dL. The yes-T2DM group included subjects with a FPG level ≥126 mg/dL, as well as those with a claims history for antidiabetic medication regardless of FPG level. The yes-T2DM group was divided into new-onset T2DM and T2DM groups according to the claims history for antidiabetic medication. Subjects who had a FPG ≥126 mg/dL and no claims history for antidiabetic medication were assigned to the new-T2DM group. Subjects who had a claims history for antidiabetic medication were assigned to the T2DM group.
Statistical analysis
Patient baseline characteristics are presented as mean±standard deviation (SD) for continuous variables, and number and percentage for categorical variables. Normally distributed continuous variables are expressed as mean±SD and were investigated by analysis of variance. Categorical variables were examined using the chi-square test. Subjects were categorized into four groups according to the duration of T2DM. The IRs of AC were calculated by dividing the number of incident cases by the total follow-up duration and are expressed as the incidence of AC per 1,000 person-years. The association between the duration of T2DM and risk of AC was evaluated with Cox proportional hazard regression models. Model 2 was adjusted for age and sex, and model 3 for age, sex, smoking status, alcohol consumption, regular exercise, hypertension, dyslipidemia, and body mass index (BMI). Results are presented as HRs with 95% confidence intervals (CIs) using the normal group as a reference. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and P<0.05 was considered to indicate significance.
RESULTS
Baseline characteristics
The analysis included 3,471,745 members of the general Korean population. During the mean follow-up of 7.9±1.54 years, 293,188 individuals (9.34%) had at least three hospital or private clinic visits, where they received the ICD-10 diagnostic code for AC (M750). Table 1 presents the baseline characteristics of the participants according to the presence of AC; those with AC were significantly older and had increased waist circumference, BMI, fasting glucose, systolic and diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, and triglyceride values. Among the AC patients, there was also a higher proportion of females, and higher IRs of DM, hypertension, dyslipidemia, and chronic kidney disease (all P<0.001). Table 2 presents IRs and HRs of AC according to subgroup of T2DM and variables. Regardless of variables, IRs and HRs of AC increased with duration of T2DM.
Overall IRs and HRs of AC according to the presence and duration of T2DM
Table 3 shows the IRs and HRs of AC according to the duration of T2DM. The IR of AC was 9.453 in the normal group, 11.912 in the prediabetes group, 14.933 in the new-T2DM group, and 24.3761 in the T2DM group. The HRs of AC was increased in the presence of T2DM. The HRs of AC after full adjustment for confounding variables (age, sex, smoking status, alcohol consumption, regular exercise, hypertension, dyslipidemia, and BMI) was 1 in the normal group, 1.084 (95% CI, 1.075 to 1.094) in the prediabetes group, 1.312 (95% CI, 1.287 to 1.337) in the new-T2DM group, and 1.473 (95% CI, 1.452 to 1.494) in the T2DM group. The secular trend of the IRs and the HRs according to duration of T2DM was statistically significant (P<0.0001) (Fig. 2). Table 4 presents subgroup analysis of IRs and HRs of shoulder AC by age and sex according to presence of T2DM. Regardless of age and sex, presence of T2DM increased the HR of AC. Interestingly, the younger adult group (age 20–29 and 30–39 years) and male group showed higher HR of AC according to presence of T2DM compared to the older adult group (age 40–49, 50–59, 60–69, 70–79, and ≥80 years) and the female group.
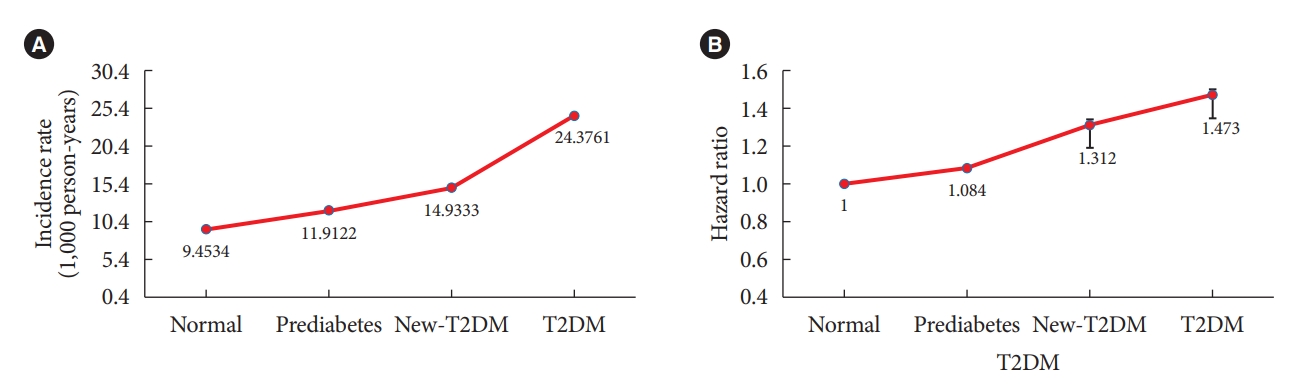
(A) Incidence rate and (B) adjusted hazard ratio of shoulder adhesive capsulitis by duration of diabetes mellitus after adjusting for age, sex, smoking status, alcohol consumption, regular exercise, hypertension, dyslipidemia, and body mass index. All P for interaction <0.001. T2DM, type 2 diabetes mellitus.
DISCUSSION
This large-scale, longitudinal, nationwide, population-based cohort study of 3,471,745 subjects demonstrated that the risk of AC increased in the prediabetes group and was associated with T2DM status. The IRs and HRs of AC increased with duration of T2DM regardless of variables. The IRs and HRs of AC were highest in the T2DM group, which included subjects who had a claims history for antidiabetic medication. In subgroup analysis, presence of T2DM increased the HR of AC regardless of age and sex. However, the younger adult group and male group showed higher HRs of AC according to presence of T2DM compared to the older adult group and the female group.
Among the components of metabolic syndrome, DM has been established as a risk factor for AC [2,9,17-21]. In one large-scale cohort study evaluating the risk of AC in a DM cohort, Lo et al. [9] reported that the incidence of AC was 3.08 times that of the comparison cohort (146.9 vs. 47.7 per 10,000 person-years). Also, the HRs of AC for diabetic patients remained significantly higher than that for non-diabetic participants (P<0.001). The prevalence of AC (12.4%) in their DM subjects is between those found in T1DM (10.3%) and T2DM (22.4%). However, their study included far fewer subjects than ours. A total of 5,109 DM cases and 20,473 reference patients were analyzed in their study, which did not exclude T1DM subjects.
The exact cause and pathology of AC is still unknown. Neviaser and Neviaser [2] reported that biopsies of the capsule showed a chronic absence of synovial lining, inflammatory infiltrate, and subsynovial fibrosis. They also found perivascular lymphocytic reactions. However, biopsy specimens from patients in the first stage of AC demonstrated a clear progression from perivascular mononuclear inflammatory infiltrates to reactive fibrosis of the capsule, which suggests an inflammatory origin [2,22]. Elevated levels of transforming growth factor-β and other profibrotic cytokines were present in capsular biopsy specimens and probably have the potential to trigger progression [23].
One hypothesis is that the hyperglycemia associated with DM can induce collagen changes within the joints [11,12]. These changes in the collagen matrix ultimately trigger the fibrotic and inflammatory alterations seen in pathologic and histochemical studies of the disease [3,13]. In addition, Esposito et al. [5] reported that hyperglycemia itself is a pro-inflammatory state. Several studies observed fibrosis induction via the proliferation of several cell types [24,25], as well as alterations in the quality and quantity of the extracellular matrix in a variety of DM patients’ tissues [26]. Increased expression of vascular endothelial growth factor and angiogenesis in diabetes-associated AC has also been observed [27]. Previous reports also suggest an immunological pathogenesis of AC and DM [24,28-31]. It is possible that these two conditions are related.
To the best of our knowledge, this study is the first nationwide, population-based cohort study to investigate the link between T2DM status (including prediabetes) and AC. Our results revealed that prediabetes increases the risk of AC. Several studies showed that the duration of T2DM can affect the progression of diabetic osteopathy, and some studies reported a link between the duration of T2DM and fractures [32,33]. However, no study has reported an association between the duration of T2DM and AC. There were two case series studies which investigated the relationship between prediabetes and AC. Tighe and Oakley [7] reported that among 88 AC patients, the prevalence of DM in patients with AC was 38.6% (34 of 88) and that of prediabetes was 32.95% (29 of 88). Thus, they insisted that practitioners should consider the risk of DM and prediabetes in patients presenting with AC. On the other hand, another case series of 50 AC patients reported an opposite relationship between prediabetes and AC [34]. That study enrolled patients with a diagnosis of idiopathic AC and no known previous diagnosis of DM or prediabetic conditions. They underwent a 2-hour oral glucose tolerance test. Four patients with idiopathic frozen shoulder (8%) were found to be prediabetic and no patients were diabetic. All four patients reported a history of DM in their parents or siblings. The authors concluded that patients diagnosed with idiopathic AC shoulder who are 60 years or younger and are not known DM have a similar probability of having DM or prediabetes to an age-matched population. So they recommended no routine diabetic workup is warranted specifically for those patients. The major limitation of both studies was the small sample size. Therefore, the findings may not be generalizable to all populations.
This study had several important strengths. First, it was a large-scale, nationwide, population-based cohort study enrolling 3,471,745 normal, 782,314 prediabetic, and 237,641 diabetic subjects. This large number of subjects ensured sufficient statistical power. Second, data from the NHIS, in which all citizens of the Republic of Korea are mandatorily enrolled, were analyzed, so there is little possibility of selection bias. Third, the prediabetes group was analyzed separately, where the association between prediabetes and AC has been rarely assessed.
There were several limitations to our study, such that the results should be interpreted with caution. First, our study only analyzed data from citizens of the Republic of Korea. Therefore, our results may not be generalizable to the worldwide population. Second, we used ICD-10 codes to identify patients with AC; therefore, patients who did not visit a hospital were not included in this study. Third, no information about shoulder range of motion was available, so we could not assess the association between diabetic status and AC severity. Fourth, we could not analyze the severity of T2DM, which can influence musculoskeletal health. Glycosylated hemoglobin (HbA1c), insulin use, and the type of antidiabetic medication were not considered in this study. Although, HbA1c of 5.7% to 6.4% is considered as prediabetes, we could not include these data. Fifth, comorbidities were not recorded. Despite adjusting for variables including hypertension, dyslipidemia, BMI, and health-related behaviors, various medical conditions could impact musculoskeletal health and the severity of T2DM such as steroid use, pancreatic disease, and other endocrinologic and rheumatologic diseases.
In summary, this large-scale, longitudinal, nationwide, population-based cohort study of 3,471,745 subjects demonstrated that the risk of AC increases in prediabetic patients and is associated with T2DM status. This finding suggests that promoting musculoskeletal health should be emphasized in prediabetic and diabetic patients care plans.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A2C2013890).
AUTHOR CONTRIBUTIONS
Conception or design: J.H.K., H.S.K., K.H.
Acquisition, analysis, or interpretation of data: K.H., B.S.K.
Drafting the work or revising: J.H.K., H.S.K., K.H.
Final approval of the manuscript: J.H.K., H.S.K., K.H.
Acknowledgements
None