Relationships between Islet-Specific Autoantibody Titers and the Clinical Characteristics of Patients with Diabetes Mellitus
Article information
Abstract
Background
Dysimmunity plays a key role in diabetes, especially type 1 diabetes mellitus. Islet-specific autoantibodies (ISAs) have been used as diagnostic markers for different phenotypic classifications of diabetes. This study was aimed to explore the relationships between ISA titers and the clinical characteristics of diabetic patients.
Methods
A total of 509 diabetic patients admitted to Department of Endocrinology and Metabolism at the Affiliated Hospital of Nantong University were recruited. Anthropometric parameters, serum biochemical index, glycosylated hemoglobin, urinary microalbumin/creatinine ratio, ISAs, fat mass, and islet β-cell function were measured. Multiple linear regression analysis was performed to identify relationships between ISA titers and clinical characteristics.
Results
Compared with autoantibody negative group, blood pressure, weight, total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), visceral fat mass, fasting C-peptide (FCP), 120 minutes C-peptide (120minCP) and area under C-peptide curve (AUCCP) of patients in either autoantibody positive or glutamate decarboxylase antibody (GADA) positive group were lower. Body mass index (BMI), waist circumference, triglycerides (TGs), body fat mass of patients in either autoantibody positive group were lower than autoantibody negative group. GADA titer negatively correlated with TC, LDL-C, FCP, 120minCP, and AUCCP. The islet cell antibody and insulin autoantibody titers both negatively correlated with body weight, BMI, TC, TG, and LDL-C. After adjusting confounders, multiple linear regression analysis showed that LDL-C and FCP negatively correlated with GADA titer.
Conclusion
Diabetic patients with a high ISA titer, especially GADA titer, have worse islet β-cell function, but less abdominal obesity and fewer features of the metabolic syndrome.
INTRODUCTION
Islet-specific autoantibodies are key markers of type 1 diabetes mellitus (T1DM) related autoimmunity and their titers are measured in variety of clinical and research settings, including for the diagnosis of T1DM [1]. Currently, four proteins have been confirmed to be autoantigens for islet-specific autoantibodies: insulin [2], glutamic acid decarboxylase [3], the protein tyrosine phosphatase-like protein IA-2 [4], and zinc transporter 8 [5].
Tuomi et al. [6] proposed a subtype of T1DM that was named latent autoimmune diabetes in adult (LADA). Patients with LADA have a similar genetic background and immune characteristics to those with T1DM, and it is characterized by slowly-progressing immune-mediated β-cell pathology and decline in β-cell function. Because the loss of β-cell function is slower than in T1DM, the early clinical characteristics of LADA are similar to those of type 2 diabetes mellitus (T2DM). At present, the diagnostic criteria of LADA remain controversial. Currently, the most recognized diagnostic criteria are those of the International Diabetic Immunology Society (IDS) [7], which comprise (1) adult onset; (2) islet autoantibody-positivity; (3) lack of insulin dependence for at least 6 months after diagnosis. As specific markers of immune destruction of islet β-cells, islet autoantibody can be used to distinguish LADA from T2DM. These antibodies include islet cell antibody (ICA), insulin autoantibody (IAA), glutamate decarboxylase antibody (GADA), protein tyrosine phosphatase antibody (IA-2A), and zinc transporter 8 antibody (ZnT8A) [8].
A study by Tuomi et al. [9] found that the metabolic syndrome is more common in patients with LADA than classic T1DM. In addition, a study conducted in Spain showed that the obesity parameters, blood pressure and the circulating triglyceride (TG) concentration in patients with LADA are higher than in those with T1DM, but lower than in those with T2DM [10]. These studies suggest that diabetic patients with positive islet autoantibodies have fewer metabolic defects than patients with negative antibodies. Lohmann et al. [11] proposed the concepts of LADA-1 and LADA-2 in 2001. The clinical characteristics of LADA patients with positive GADA, ICA, and high titer GADA are phentotypically closer to patients with T1DM, which is called LADA-1 type. In contrast, patients who clinically more closely resemble T2DM patients, are positive for a single autoantibody, and have low autoantibody titers, are classified as having LADA-2. Recent studies have shown that positive autoantibody titer, especially GADA titer, are realted to the clinical characteristics of patients with LADA [121314]: LADA patients with a high GADA titer (LADA-1) have characteristics more similar to T1DM than T2DM (low C-peptide concentration, low body mass index [BMI], and a greater predisposition toward ketosis); whereas patients with a lower GADA titer (LADA-2) are less likely to develop ketosis than patients with LADA-1, and the prevalences of obesity, hypertension, dyslipidemia, and cardiovascular disease are higher. Similar results were also obtained in a Korean study, in which GADA titer negatively correlated with the age of onset, total cholesterol (TC) and TG concentrations, BMI, and fasting and postprandial C-peptide concentrations; and positively correlated with glycosylated hemoglobin (HbA1c) and high density lipoprotein cholesterol (HDL-C) concentration [15].
A number of studies have confirmed an inverse relationship between GADA titer and C-peptide concentration [16], and patients with higher GADA titers show more insulin deficiency [13]. Not only the different autoantibody titers, but also the different phenotypes of positive autoantibody will affect the clinical characteristics of LADA patients. Among a group of about 17,000 subjects affected by LADA, only the subjects with IA-2A positive will show a more similar clinical phenotype to T2DM, while the subjects with IA-2A and GADA positive will show a more similar clinical phenotype to T1DM [17]. Although islet autoantibodies have been widely used for the diagnosis of diabetes, most studies of islet autoantibodies conducted in China have been qualitative, with few studies of autoantibody titers having been published. Therefore, it remains to be determined whether the autoantibody titer is related to metabolic parameters and islet function. In the present study, we aimed to determine the relationships between the types and titers of islet-specific autoantibodies and the clinical characteristics of diabetic patients.
METHODS
Participants
From November 2017 to October 2018, a total of 509 diabetic patients admitted to the Department of the Endocrinology and Metabolism at the Affiliated Hospital of Nantong University were recruited. Diabetes was diagnosed according to the World Health Organisation diagnostic criteria (1999).
The exclusion criteria were: (1) age <18 years; (2) patients with other types of diabetes: gestational diabetes, drug-induced diabetes, pancreatic exocrine diseases, and other endocrine diseases such as hyperthyroidism; (3) patients with severe acute complications of diabetes, severe liver or kidney dysfunction, autoimmune diseases, malignant tumors, and women during pregnancy and lactation; (4) patients with primary renal disease and eye disease; (5) patients with mental illness.
This study was approved by the Ethics Committee of Affiliated Hospital of Nantong University (ethics No. 2018-k016). All patients gave their written informed consent.
Clinical characteristics
General information was collected using a questionnaire, including age, gender, duration of diabetes, previous medical history (especially with regard to hypertension and coronary heart disease), family history of diabetes, medication status. The height, weight and waist circumference of the participants were measured. BMI (kg/m2) was calculated by dividing weight (kg) by height squared (m2). Blood pressure was measured using an HEM-7200 (Omron, Kyoto, Japan) electronic sphygmomanometer.
Laboratory measurements
Venous blood samples were collected in the morning after an overnight fast of 12 hours. The serum TC, TG, low density lipoprotein cholesterol (LDL-C), HDL-C, uric acid (UA), and creatinine (Cr) concentrations were measured using an ADVIA2400 automated biochemical analyzer (Siemens, Tarrytown, NY, USA). HbA1c was measured using a Variant TM II hemoglobin analyzer (Bio-Rad, Hercules, CA, USA). Islet-specific autoantibody titers were measured using a Yahuilong 3000 iFlash chemiluminescence immunoassay (Shenzhen Yahuilong Biotechnology, Shenzhen, China). A random urine sample was obtained from each participant to determine the urinary microalbumin concentration (BioSystems A25 automatic specific protein analyzer; BioSystems, Barcelona, Spain) and to calculate the urinary microalbumin/creatinine ratio. The MDRD formula was used to calculate the estimated glomerular filtration rate (eGFR): eGFR (mL/min/1.73 m2)=186×(Cr)−1.154×(age)−0.203 (×0.742 if female).
Each participant underwent an insulin release test in which they consumed 100 g of steamed bread on an empty stomach in the morning, then venous blood was collected 0, 30, 60, 90, 120, 150, and 180 minutes later for the measurement of blood glucose, and serum insulin and C-peptide concentrations. Insulin and C-peptide were measured using an e411 automated electrochemical luminescence immunoanalyzer (Roche, Tokyo, Japan).
Each of the participants were screened for diabetic retinopathy and diabetic nephropathy. Diabetic retinopathy [18] was diagnosed according to the criteria of the International Clinical Classification of Diabetic Retinopathy (2002). Diagnostic criteria for diabetic nephropathy [19]: eGFR <60 mL/min/1.73 m2; the urinary microalbumin creatinine ratio was reviewed within 3 to 6 months, and the excretion of urinary protein increased in two of the three times (urine albumin/creatinine ratio [UACR] ≥30 mg/g).
Whole body fat mass and visceral fat mass was measured using dual energy X-ray absorptiometry (Prodigy; GE Healthcare, Madison, WI, USA).
Assessment of β-cell function and insulin resistance
Homeostatic model assessment was used to estimate basal β-cell function (HOMA-β) and insulin resistance (HOMA-IR). HOMA-β=20×fasting insulin (FINS; mIU/L)/[fasting plasma glucose (FPG; mmol/L)−3.5]; HOMA-IR=FPG (mmol/L)×FINS (mIU/L)/22.5 [20].
The Matsuda index, which reflects systemic insulin sensitivity, was also calculated: Matsuda index=10,000/(FPG×FINS×mean blood glucose×mean insulin)1/2 [21]. Early islet β-cell secretory function was assessed using the early-phase insulin secretion index (ΔI30/ΔG30) [22], in which ΔI30 and ΔG30 represent the increase in insulin concentration and glucose concentration in the 30 minutes following the glucose load, respectively.
The area under the curve (AUC) was calculated using the irregular trapezoidal rule: AUC=15×fasting value+30×(30 min value+180 min value)+45×(60 min value+60×120 min value). The AUCINS, AUCGlu, and AUCCP represent the areas under the insulin, glucose, and C-peptide curves, respectively. AUCINS/AUCGlu was used to evaluate insulin secretion within the 180 minutes period [23].
Glucose disposition index (DI) was also used to assess islet β-cell function: DI=ΔI30/ΔG30 (AUCINS/AUCGlu)×1/HOMA-IR [24].
Autoantibody grouping
The cut-off values of islet-specific autoantibody titers denoting positivity were: GADA titer ≥10 IU/mL, IAA titer ≥1.0 IU/mL, and ICA titer ≥1.0 IU/mL. According to their titers, the participants were allocated to GADA positive and GADA negative groups. According to the number of islet autoantibodies (ICA, GADA, IAA) that each participant was positive for, they were also allocated to either autoantibody positive group (positive for ≥1 autoantibody) and autoantibody negative group.
Statistical analysis
SPSS version 20.0 software (IBM Co., Armonk, NY, USA) was used for statistical analysis. Normally-distributed continuous data was expressed as mean±standard deviations, non-normally-distributed continuous data was expressed as median and interquartile ranges, and categorical data are expressed as numbers and percentages. The independent-samples t-test was used to compare the normally-distributed continuous data between the two groups. For non-normally-distributed continuous data, non-parametric tests were used for comparison between groups. Pairwise comparisons among multiple groups were performed using a non-parametric test. Comparisons between categorical datasets were conducted using the chi-square test. Spearman correlation analysis was used to analyze the relationships between autoantibody titers and other parameters. The factors influencing islet autoantibody titer were identified using multiple linear regression analysis. Differences were considered significant when P<0.05.
RESULTS
Positive distribution of islet autoantibodies
In this study, the distribution of islet autoantibodies among the participants is shown in Table 1. The positive rate of GADA, IAA, ICA, and all three were 17.9%, 12.3%, 29.3%, and 4.9%, respectively.
Comparison of the clinical characteristics between either autoantibody positive group and autoantibody negative group
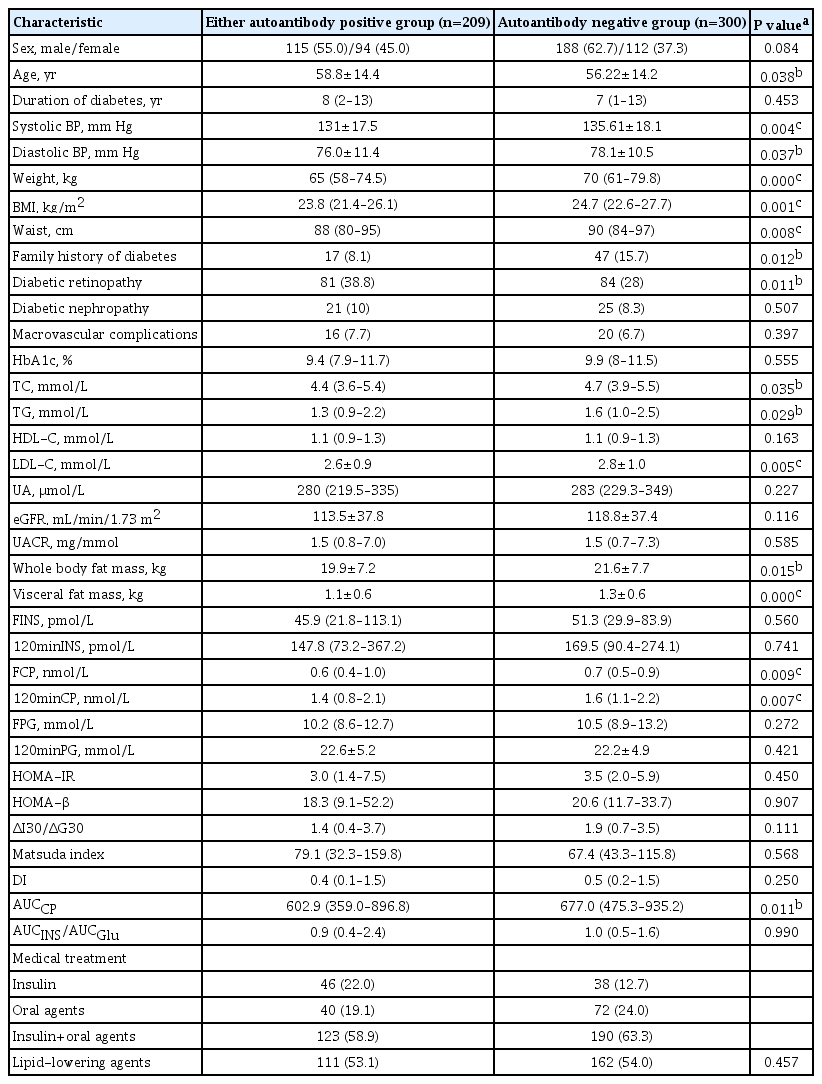
All the participants were grouped according to their islet autoantibody test results. There were 209 participants in either autoantibody positive group and 300 participants in autoantibody negative group. The clinical characteristics of the two groups were compared in Table 2. There were no significant differences in gender composition, duration of diabetes, prevalence of diabetic nephropathy, prevalence of macrovascular complications, HbA1c, HDL-C, UA, eGFR, UACR, FINS, 120 minutes insulin (120minINS), FPG, 120 minutes plasma glucose (120minPG), HOMA-IR, HOMA-β, ΔI30/ΔG30, Matsuda index, DI, or AUCINS/AUCGlu between the two groups (all P>0.05). Compared with the autoantibody negative group, the average age of patients in either autoantibody positive group was larger, with lower systolic and diastolic blood pressure, lower body weight, smaller BMI and waist circumference. In addition, TC, TG, LDL-C, body fat mass, visceral fat mass, fasting C-peptide (FCP), 120 minutes C-peptide (120minCP), and AUCCP were all lower than those in the autoantibody negative group, and the differences were statistically significant (all P<0.05). The rate of family history of diabetes in either autoantibody positive group was lower than that in all autoantibody negative group (8.1% vs. 18.7%, P=0.012), and the incidence of diabetic retinopathy was higher than that in all autoantibody negative group (38.8% vs. 28%, P=0.011).
Comparison of the clinical characteristics between GADA negative group and GADA positive group
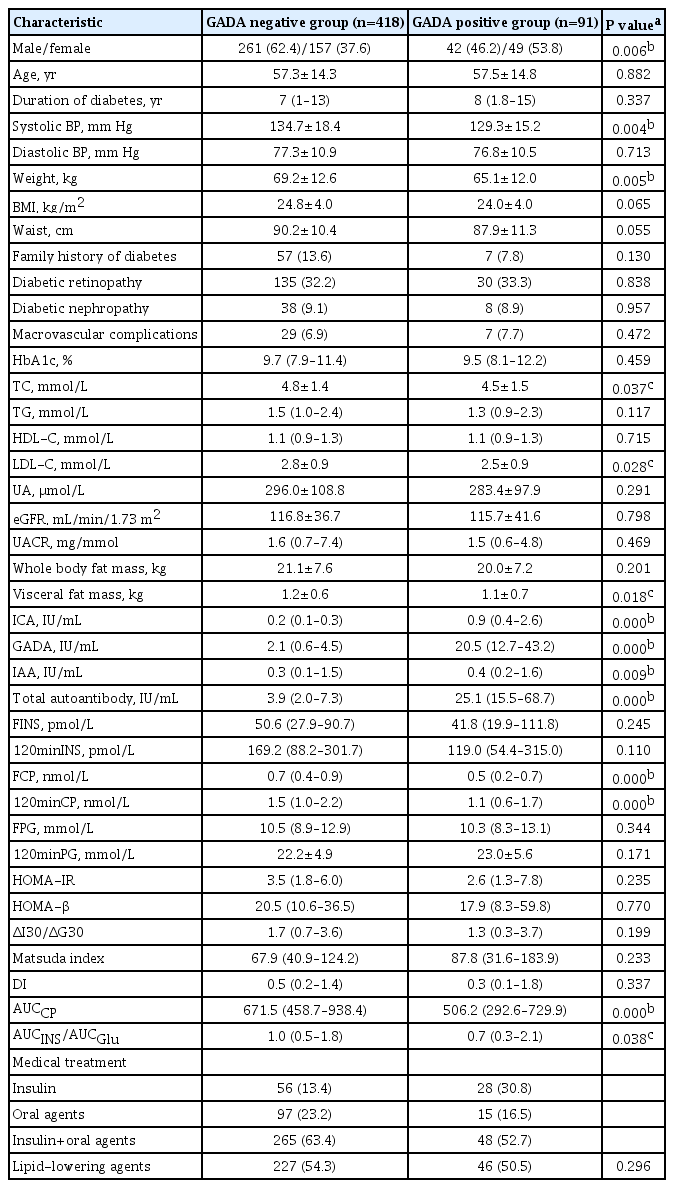
There were 418 participants in GADA negative group and 91 in GADA positive group. The comparison of clinical characteristics of the two groups is shown in Table 3. There were no significant differences in age, duration of diabetes, diastolic blood pressure, BMI, waist circumference, family history of diabetes, prevalence of diabetic retinopathy, prevalence of diabetic nephropathy, prevalence of macrovascular complications, HbA1c, TG, HDL-C, UA, eGFR, UACR, whole body fat mass, FINS, 120minINS, FPG, 120minPG, HOMA-IR, HOMA-β, ΔI30/ΔG30, Matsuda index, or DI between the two groups (all P>0.05). Compared with the GADA negative group, the GADA positive participants were less likely to be male, and had lower systolic blood pressure and body weight. TC, LDL-C, visceral fat mass, FCP, 120minCP, AUCCP, and AUCINS/AUCGlu were all significantly lower in the GADA positive than in the GADA negative group, and the ICA and IAA titers were significantly higher (all P<0.05).
Correlation analysis
Analysis of the relationships between GADA titer and various clinical parameters showed that the GADA titer level of diabetic patients was positively correlated with ICA titer (r=0.458, P=0.000), and was negatively correlated with TC (r=−0.116, P=0.009), LDL-C (r=−0.088, P=0.047), FCP (r=−0.152, P=0.001), 120minCP (r=−0.144, P=0.001), AUCCP (r=−0.14, P=0.002), but there were no significant correlations with the other parameters (Table 4).
Analysis of the relationships between ICA titer and various clinical parameters showed that the ICA titer level of diabetic patients was positively correlated with age, GADA titer, IAA titer (all P<0.05), and was negatively correlated with body weight, BMI, waist circumference, TC, TG, LDL-C, eGFR, visceral fat mass, FCP, 120minCP, and AUCCP (all P<0.05), but there were no significant correlations with other parameters (Table 4).
Analysis of the relationships between IAA titer and various clinical parameters showed that the IAA titer level of diabetic patients was positively correlated with age, duration of diabetes, incidence of diabetic retinopathy, ICA titer (all P<0.05), and was negatively correlated with diastolic BP, body weight, BMI, HbA1c, TC, TG, and LDL-C (all P<0.05), but there were no significant correlations with other parameters (Table 4).
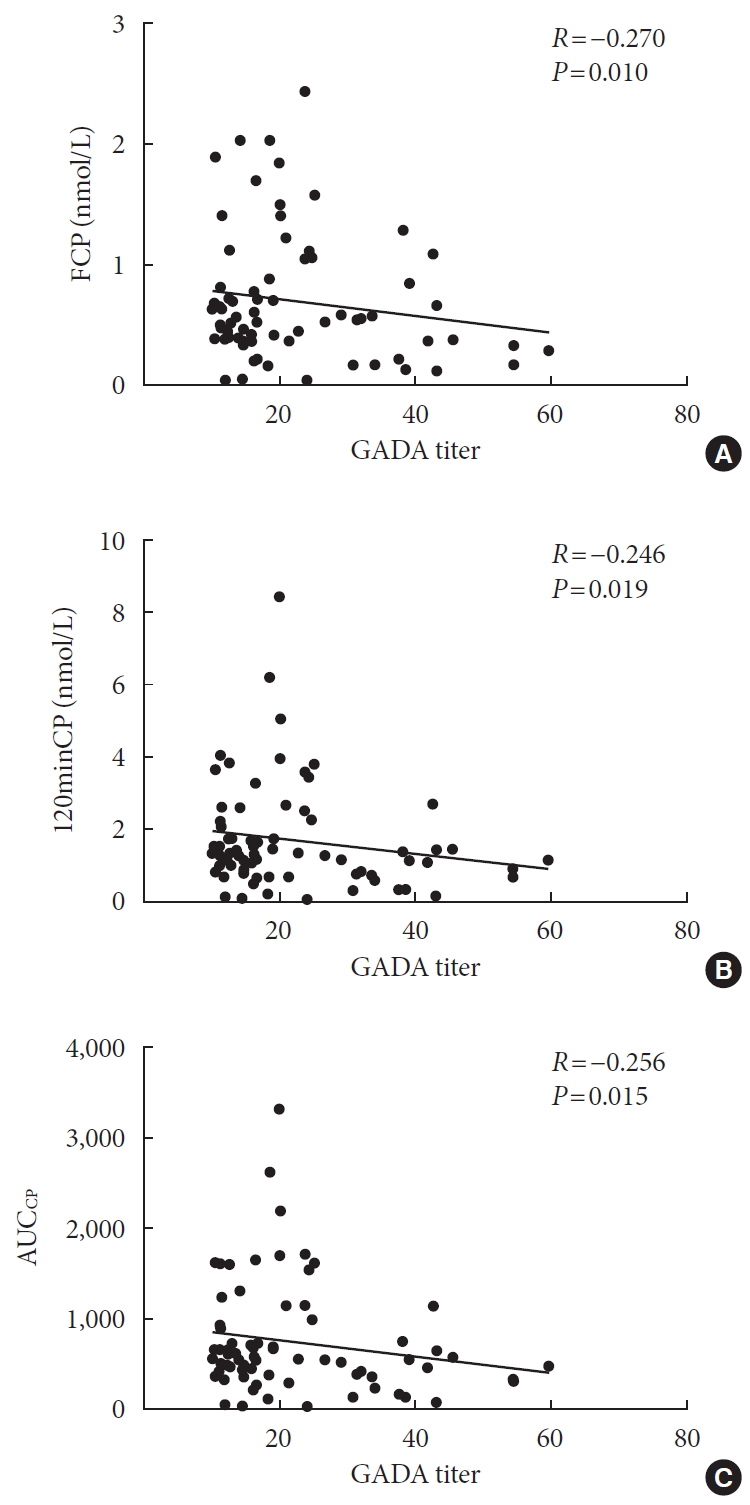
After excluding GADA negative participants, the relationships between GADA titer and FCP, 120minCP and AUCCP was then analyzed. The results showed that GADA titer level in GADA positive group was negatively correlated with FCP (r=−0.270, P=0.010), 120minCP (r=−0.246, P=0.019), AUCCP (r=−0.256, P=0.015) (Fig. 1).
Multiple linear regression analysis of the influencing factors of the GADA, ICA, and IAA titers
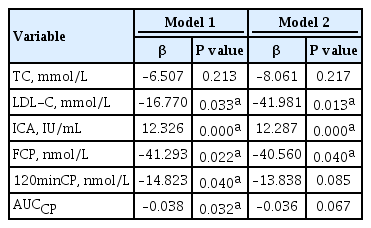
After adjustment for potential confounders, multiple linear regression analysis was performed on GAD, ICA, and IAA titers. The results showed that when GADA titer was used as the dependent variable, LDL-C and FCP were independently negatively correlated with GADA titer, and ICA titer was independently positively correlated with GADA titer (Table 5). When ICA titer was used as the dependent variable, there were independent positive correlations among the GADA, IAA, and ICA titers (Supplementary Table 1). When IAA titer was used as the dependent variable, the duration of diabetes independently negatively correlated with IAA titer, and ICA titer independently positively correlated with IAA titer (Supplementary Table 2).
DISCUSSION
Positive rate of islet autoantibodies
Since the late 1970s, evidence of the presence of circulating autoantibodies in adult non-insulin-dependent diabetes has emerged [625]. Turner et al. [26] reported consistent evidence of islet cell autoimmunity in T2DM patients in 1997, with more than 3,000 T2DM patients between the ages of 25 and 65 recruited at the United Kingdom Prospective Diabetes Study (UKPDS) trial center, GADA and ICA were found in 12% of patients; 12% of patients over the age of 65 with T2DM phenotype detected GADA and/or IA-2A [27]. In this study, we measured the titers of GADA, ICA, and IAA only, because of the experimental conditions. The prevalences of GADA, ICA, and IAA were 17.9% (91/509), 12.3% (63/509), and 29.3% (149/509), respectively. In the study of large sample populations in China, Huang et al. [28] found that the prevalences of GADA was 5.9% and the prevalences of IAA was 3.39%. In this study, the positive rate of GADA and IAA was significantly higher than that reported by Huang et al. [28], which may be due to different detection methods used or the influence of the use of insulin on the generation of IAA. In this study, 76.5% of IAA-positive patients were treated with insulin, which may be one of the reasons for high prevalence of IAA.
Islet autoantibodies and metabolic characteristics
The European Action LADA multicenter study found that the prevalence of metabolic syndrome in LADA patients was similar to that in T1DM patients, lower than that in T2DM patients [29]. Similarly, a multicenter study conducted in China found that the prevalence of metabolic syndrome in LADA patients was slightly lower than in T2DM patients, but higher than in T1DM patients [30]. Li et al. [31] found that LADA-1 patients tended to be thinner, have higher autoantibody titers and fewer features of the metabolic syndrome, whereas LADA-2 patients were similar to T2DM patients, being autoantibody positive but having low titers and more features of metabolic syndrome. Taken together, the results of these studies suggest that islet autoantibody titer is closely related to the metabolic status of diabetic patients.
In the present study, we analyzed the difference in metabolic parameters between either autoantibody positive and autoantibody negative participants. The results showed that participants who were positive for any autoantibody (GADA, ICA, or IAA) tended to be older than those who were not, and had lower blood pressure,weight, BMI, waist circumference, blood lipids, and body fat mass. Thus, islet autoantibody positive patients are more likely to be older, thinner, and to have lower circulaiting lipid concentrations. Similarly, the blood pressure, body mass, blood lipid concentrations, and visceral fat mass of the GADA positive group were lower than those of the GADA negative group, which is consistent with previous findings. Therefore, early screening for islet autoantibodies may be useful in diabetic patients with these characteristics, to improve the diagnostic success for LADA.
We also performed correlation analyses which showed that the ICA and IAA titers negatively correlated with body mass, BMI, TC, TG, and LDL-C. Therefore, we can speculate that the higher the ICA or IAA titer is, the less likely the patient is to develop metabolic syndrome. Correlation analysis of GADA titer showed positive correlation with ICA titer and negative correlation with TC and LDL-C. Multiple linear regression analysis was performed after correction for potential confounding factors, which showed that LDL-C was negatively correlated with GADA titer, and ICA titer was positively correlated with GADA titer. However, multiple linear regression analysis of the ICA and IAA titers showed that they did not independently correlate with any metabolic parameters. This indicates that GADA titer is most closely related to lipid metabolism among the three islet autoantibodies studied.
Islet autoantibodies and islet β-cell function
Several studies have confirmed that GADA titers are inversely related to C-peptide concentration [16]. UKPDS [32] showed that GADA can predict islet function in LADA patients. Further studies have found that patients with low GADA titers have a slower decline in islet function, while patients with high GADA titers have a faster decline in islet function. Therefore, GADA titers are considered to predict changes in islet function decline in patients [33]. Early recognition of GADA-positive (especially high GADA titers) and early intervention therapy can significantly delay the decline in islet function. In the present study, we found that patients with positive antibodies in any of GADA, ICA, and IAA had lower FCP, 120minCP, and AUCCP than patients lacking these autoantibodies. In addition, the FCP, 120minCP, AUCCP, and AUCINS/AUCGlu in GADA positive group were lower than those in the GADA negative group, suggesting that the presence of any islet autoantibody, especially GADA, predicted worse islet function. In addition, this study also found that with the increase of FCP, the patient's ICA and GADA titers showed a trend of decline, indicating that the better the islet function, the lower the positive titer of islet autoantibodies, so islet autoantibody titer can effectively reflect the islet function in diabetic patients. This finding is consistent with those of previous studies, and together, these findngs imply that early detection of islet autoantibodies can be used to predict islet function and suggest the need for early intervention to protect function. Further analysis of the correlation between GADA titer and islet function related parameters showed that GADA titer negatively correlated with FCP, 120minCP, and AUCCP. The results of multiple linear regression analysis indicated that the FCP independently negatively correlated with the GADA titer, confirming that C-peptide concentration tends to be lower when GADA titer is high.
The prevalence of ICA in this study is low (12.3%). IAA represents a group of antibodies against insulin that can prevent insulin from having its biological effects. The half-life of these antibodies is short, and their prevalence decreases with the increase of age. Current detection methods cannot distinguish endogenous IAA from antibodies produced after insulin application, so IAA is limited in clinical application. In this study, the positive rate of IAA was relatively high due to the failure to exclude patients treated with insulin. The results of correlation analysis of IAA showed that the IAA titer positively correlated with the duration of diabetes. The reason may be that with the longer duration of diabetes, more patients needed insulin therapy, which caused the IAA titer increased subsequently.
Islet autoantibodies and vascular complications
There is no consistent opinion on the differences of microvascular complications between T1DM, T2DM, and LADA patients, which may be due to heterogeneity in previous studies with respect to sample size, ethnicity, and disease duration. Isomaa et al. [34] compared the complications in patients with long-standing LADA, T2DM, or T1DM. The results showed that the prevalence of microvascular complications in LADA patients was similar to that in classic T1DM patients, and the prevalence of retinal and renal diseases was not significantly different from that in T2DM patients. Other studies have compared the prevalences of complications in patients with LADA or T2DM of short duration, and showed that the prevalence of microalbuminuria is lower in patients with LADA of short duration than in T2DM patients [35]. In a cross-sectional study conducted in China, the prevalences of nephropathy and retinopathy in LADA patients with a disease duration of less than 5 years was observed to be lower than in patients with T2DM, but no difference was observed in patients with a disease duration more than 5 years [36]. This may be due to the fact that T2DM patients are usually diagnosed later than LADA patients, and prolonged exposure to hyperglycemia increases the incidence of microvascular complications. As for the correlation between autoantibody titers and chronic complications of LADA, Jensen et al. [37] studied and found that LADA patients with high GADA titers had an increased risk of diabetic retinopathy after follow-up for 15 years.
In this study, compared with the antibody negative group, the prevalence of diabetic retinopathy was higher in either antibody positive group, but there was no significant difference in the duration of diabetes or the prevalence of macrovascular complications between the two groups. Furthermore, there were no differences in the prevalences of diabetic nephropathy, diabetic retinopathy, or macrovascular complications between GADA positive and negative participants, which may be because of the relatively short duration of diabetes and the smaller sample size, or the relatively close correlations between ICA, IAA and the prevalence of microvascular complications. Further prospective studies are needed to investigate the relationship between islet autoantibodies and microvascular complications.
Limitations
This is a one-center cross-sectional study. It can only observe the correlation between islet autoantibodies and clinical characteristics, failing to judge the causal relationship, which needs to be verified by further prospective studies. Previous studies have found that LADA is associated with human HLA-II genes, but we were unable to sequence these genes in the present study participants. Future studies should involve the genetic testing of participants, in order to further explore the relationship between islet autoantibodies and gene expression.
The most widely recognized diagnostic criteria for LADA are currently those of the IDS [7]: (1) adult onset; (2) islet autoantibody-positivity; (3) lack of insulin dependence for at least 6 months after diagnosis. Since the subjects included in this study were all inpatients with poor blood glucose control, and a large proportion of patients had already started insulin therapy before the islet autoantibody test, so it is difficult to distinguish between these types of patients. The clinical phenotype of LADA is highly heterogeneous, combining the characteristics of T1DM and T2DM, which can be expressed as classic T1DM with absolute insulin deficiency, or as T2DM with obesity and insulin resistance [38]. Therefore, it would not be appropriate to distinguish the type of diabetes according to the clinical phenotype alone. Due to the small sample size, in order to facilitate statistical analysis, participants in this study were not classified according to T1DM, T2DM and LADA. Therefore, specific classification of participants can be conducted on the basis of expanding sample size to explore relevant factors of islet autoantibody titer in different types of diabetes.
Future prospects
There may be other islet-related autoantibodies that have not been identified yet. In the future, ZnT8A and other newly discovered antibodies should also be studied. Long-term follow-up studies are required to determine whether the type of antibody or its titer can predict the rate of decline in islet function in diabetic patients.
In conclusion, diabetic patients with high titers of islet-specific autoantibodies, especially GADA titer, have worse islet β-cell function, but less abdominal obesity and fewer features of the metabolic syndrome.
ACKNOWLEDGMENTS
The current study is supported by the Jiangsu Provincial Six Talent Peaks for high-level talents (2016-WSN-098; SWYY-051); Nantong Municipal Science and Technology Project (HS2014036); the Project of Preventive Medicine Association of Jiangsu Provincial Health and Family Planning Commission (Y2015070); Nantong Municipal Science and Technology Project (MS22019005); Research on the Construction of Endocrinology Special Specialty at the Grass-roots Level (2020JCC034).
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: Y.Z., Y.G.
Acquisition, analysis, or interpretation of data: Y.Z., T.Y., Y.G.
Drafting the work or revising: Y. Z., T.Y., X.W., R.Z., J.Y., Y.S., J.Z., S.C., Y.G.
Final approval of the manuscript: Y.Z., T.Y., X.W., R.Z., J.Y., Y.S., J.Z., S.C., Y.G.
FUNDING
None
References
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2019.0239.
Supplementary Table 1
Multiple linear regression analysis of clinical measurements and ICA titer
Supplementary Table 2
Multiple linear regression analysis of clinical measurements and IAA titer