Balsamic Vinegar Improves High Fat-Induced Beta Cell Dysfunction via Beta Cell ABCA1
Article information
Abstract
Background
The aim of this study was to investigate the effects of balsamic vinegar on β-cell dysfunction.
Methods
In this study, 28-week-old Otsuka Long-Evans Tokushima Fatty (OLETF) rats were fed a normal chow diet or a high-fat diet (HFD) and were provided with tap water or dilute balsamic vinegar for 4 weeks. Oral glucose tolerance tests and histopathological analyses were performed thereafter.
Results
In rats fed both the both chow diet and the HFD, the rats given balsamic vinegar showed increased insulin staining in islets compared with tap water administered rats. Balsamic vinegar administration also increased β-cell ATP-binding cassette transporter subfamily A member 1 (ABCA1) expression in islets and decreased cholesterol levels.
Conclusion
These findings provide the first evidence for an anti-diabetic effect of balsamic vinegar through improvement of β-cell function via increasing β-cell ABCA1 expression.
INTRODUCTION
The prevalence of type 2 diabetes is continuously increasing and this results in serious health problems. Both decreased β-cell function and increased insulin resistance contribute to the onset of type 2 diabetes. This disease is closely related to many metabolic disorders such as hypertension, obesity, and hypercholesterolemia. In previous studies, hypercholesterolemia resulted not only in islet cholesterol accumulation in mice [1,2], but also caused severe β-cell dysfunction, subsequently [3].
ATP-binding cassette transporter subfamily A member 1 (ABCA1) is known as a key molecule in islet cholesterol metabolism. ABCA1 is a plasma membrane protein that mediates the efflux of cellular cholesterol in β-cells [4]. β-cells without ABCA1 are unable to degrade cholesterol. A recent study has shown that a lack of β-cell ABCA1 leads to the impaired exocytotic fusion of insulin granules [5]. This finding means that ABCA1 plays an important roll in β-cell insulin secretion.
Several studies have shown that vinegar can improve postprandial hyperglycemia and insulin resistance [6,7], although the exact mechanism is still unclear. In this study, we investigated the effect of balsamic vinegar on insulin secretion as measured by ABCA1 expression in Otsuka Long-Evans Tokushima Fatty (OLETF) rats, an animal model of type 2 diabetes.
METHODS
Experimental animals
Forty OLETF rats were obtained from the Tokushima Research Institute (Otsuka, Tokushima, Japan), and all experiments were performed in accordance with the guidelines for Animal Research from the American Association for Accreditation of Laboratory Animal Care (AAALAC) International and were approved by the Yonsei Laboratory Animal Research Center. At first, all rats were fed a normal diet during a 1-week quarantine and acclimation period. At 28 weeks of age, the rats were randomly divided into four groups (n=10 per group) and were followed for 4 weeks. The four groups were chow diet (CD)-fed rats provided with tap water, CD-fed rats provided with balsamic vinegar, high fat diet (HFD)-fed rats provided with tap water, and HFD-fed rats provided with balsamic vinegar.
Preparation of balsamic vinegar
The balsamic vinegar was purchased from a local source in Seoul (balsamic vinegar of Modena; Acetum S.R.L, Cavezzo, Italy). The crude balsamic vinegar was diluted with tap water at a ratio of 3:1. The dilute balsamic vinegar was changed daily at 10:00 AM.
Glucose tolerance test
After 4 weeks on their respective diets, an oral glucose tolerance test was performed on all groups after an overnight fast of 13 to 15 hours. Glucose (2.0 g/kg body weight) was administrated by gavage, and blood samples were obtained by tail bleeding, before and 15, 30, 60, and 120 minutes after the glucose load. Blood glucose levels were measured by an ACCU-Chek Compact instrument (Roche Diagnostics, Indianapolis, IN, USA).
Immunohistochemistry
Histological sections of pancreas (5 µm) were cut from two rats of each group. We performed immunohistochemistry with antibodies against insulin (Santa Cruz Biotechnology Inc., Heidelberg, Germany) and ABCA1 (ABR-Affinity BioReagents Inc., Golden, CO, USA) on paraffin-embedded pancreatic sections. For each pancreas, 10 sections were evaluated and quantitation of islet cell areas was performed by counting cells in islets stained with anti-insulin antibody, as previously described [8]. For the immunohistochemical staining we used an avidin-biotin-peroxidase complex staining system (Santa Cruz Biotechnology Inc.), which results in a red immunoreactive signal with a nuclear counterstain from the use of methyl green [9].
Fluorescence staining
To study the cellular distribution of cholesterol, pancreatic sections from rats were stained using filipin (Sigma, St Louis, MO, USA) and counterstained using 4', 6-diamidino-2-phenylindole (DAPI; Sigma). The pancreatic sections were fixed for 10 minutes with 4% paraformaldehyde in phosphate buffered saline (PBS) at room temperature, followed by a 35 minutes wash in PBS and a 10-minute incubation in 1.5% glycine in PBS. After three 5-minute washes in PBS, the pancreatic sections were incubated for 1 hour at room temperature with filipin (0.05 mg/mL; Sigma) in PBS. After incubation, the sections were washed and coverslipped with fluorescent mounting media (DakoCytomation, Carpinteria, CA, USA). The filipin stained experimental and control pancreata were analyzed with a Nikon TE2000U fluorescent microscope (Nikon, Tokyo, Japan).
Data analysis
The data are presented as means±standard errors of the means. We used Student's t-test to evaluate for statistically significant differences between groups. The area under the curve for glucose was calculated using the trapezoidal rule. A P value of less than 0.05 was considered statistically significant. The statistics program used for the analysis was the SPSS package for Windows version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Oral glucose tolerance test
The oral glucose tolerance test on fasted rats showed improved glucose tolerance in the balsamic vinegar-administered rats (Fig. 1A). Plasma glucose levels at 60 and 120 minutes were improved in both the CD and the HFD groups with balsamic vinegar administration. Two hours after glucose loading the plasma glucose level of the HFD group with balsamic vinegar decreased to that of the CD group with tap water. The HFD with balsamic vinegar group showed a reduced area under the curve for glucose compared with that of the HFD with tap water group (Fig. 1B).
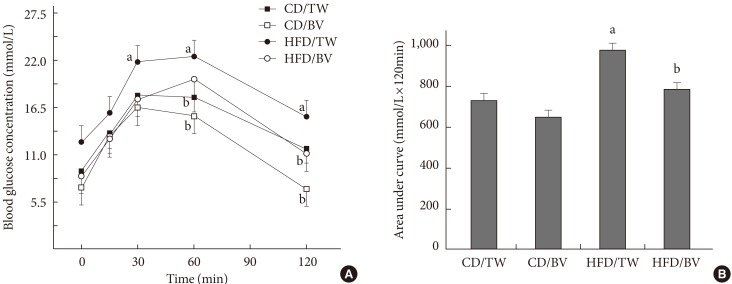
Effects of balsamic vinegar on plasma glucose levels according to the oral glucose tolerance test (OGTT). (A) The OGTT and (B) glucose area under curve were performed on Otsuka Long-Evans Tokushima Fatty rats administered water or balsamic vinegar at 31 weeks of age after a 24-hour fast. Glucose (2 g/kg) was administered orally. CD, chow diet; HFD, high-fat diet; TW, tap water; BV, balsamic vinegar. aP<0.05 compared with tap water-treated chow diet-fed rats, bP<0.05 compared with tap water-treated high fat diet-fed rats.
Islet insulin level
Insulin expression levels in the pancreatic islets of all groups are shown in Fig. 2. The islets of the HFD with tap water group demonstrated areas of decreased insulin immunoreactivity compared with those of the CD group with tap water. In the CD groups, balsamic vinegar-treated rats showed an increased insulin staining area with a 1.4-fold increase compared to tap water-treated rats. In the HFD groups, balsamic vinegar-treated rats showed an increased insulin staining area with a 1.75-fold increase compared to tap water-treated rats. These results indicate that islets from the balsamic vinegar-treated rats expressed significantly more insulin than islets from the non-treated rats in both the CD and HFD groups.
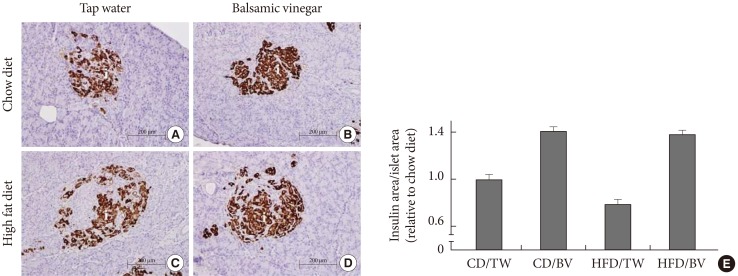
Immunohistochemical staining of representative pancreatic islets from water administered Otsuka Long-Evans Tokushima Fatty (OLETF) rats and balsamic vinegar administered OLETF rats (×200). Pancreatic sections were stained with antibodies against insulin. (A) Chow diet-fed rats administered water. (B) Chow diet-fed rats administered balsamic vinegar. (C) High-fat diet-fed rats administered water. (D) High-fat diet-fed rats administered with balsamic vinegar. (E) Insulin area/islet area. Data are mean±standard error of the mean. Scale bars are shown in the panel at 200 µm. CD, chow diet; HFD, high-fat diet; TW, tap water; BV, balsamic vinegar.
Islets cholesterol level
We examined the expression patterns of pancreatic cholesterol using filipin staining (Fig. 3). There were no significant differences in cholesterol staining between the CD with tap water group and the HFD with tap water group. With the balsamic vinegar treatment, cholesterol staining was reduced in both the CD and HFD groups.
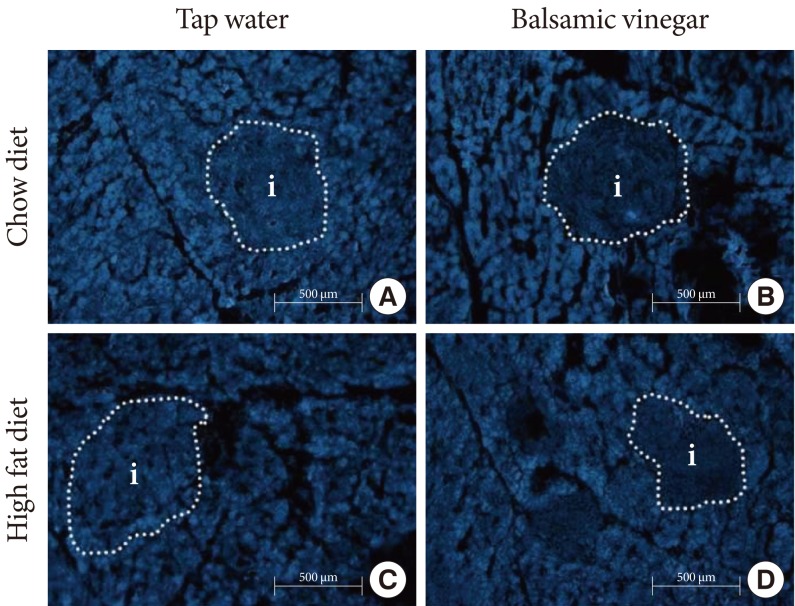
Histological sections of the pancreas from water administered Otsuka Long-Evans Tokushima Fatty (OLETF) rats and balsamic vinegar administered OLETF rats (×200). Fluorescence staining was done with filipin. Islets boundaries (i) are indicated with dotted lines. (A) Chow diet-fed rats administered water. (B) Chow diet-fed rats administered balsamic vinegar. (C) High-fat diet-fed rats administered water. (D) High-fat diet-fed rats administered balsamic vinegar. Scale bars are shown in the panel at 500 µm.
Islets ABCA1 expression
ABCA1 expression in the pancreatic islets was evaluated (Fig. 4). In the tap water treated groups, neither the CD or HFD groups showed any detectable differences in islet ABCA1 expression. With balsamic vinegar treatment, ABCA1 expression increased in the CD and HFD groups.
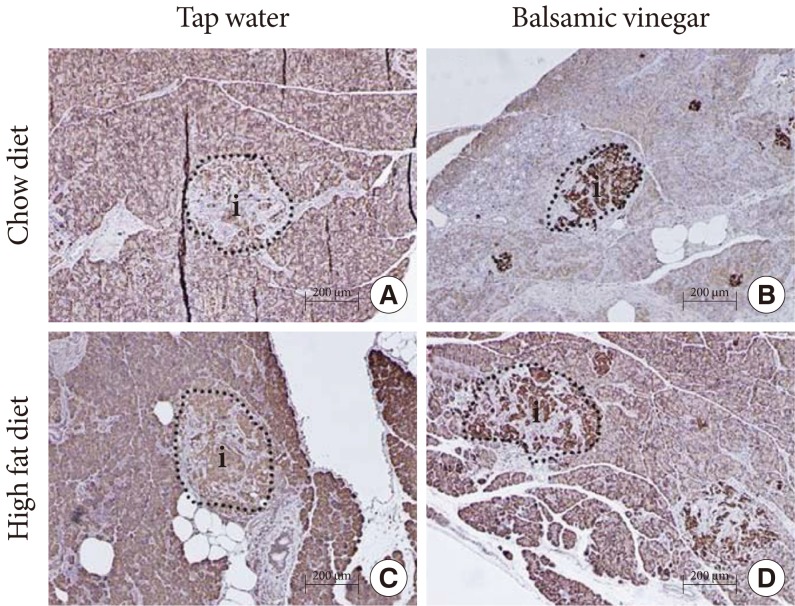
Immunohistochemical staining of representative pancreatic islets from water-administered Otsuka Long-Evans Tokushima Fatty (OLETF) rats and balsamic vinegar-administered OLETF rats (×200). Pancreatic sections were stained with antibodies against ATP-binding cassette transporter subfamily A member 1 (ABCA1). Islets boundaries (i) are indicated with dotted lines. (A) Chow diet-fed rats administered water. (B) Chow diet-fed rats administered balsamic vinegar. (C) High-fat diet-fed rats administered water. (D) High-fat diet-fed rats administered balsamic vinegar. Scale bars are shown in the panel at 200 µm.
DISCUSSION
Glucose tolerance and islet response to balsamic vinegar administration were investigated in OLETF rats. In our study, marked impairment of the insulin response was accompanied with HFD feeding. In the HFD groups, glucose tolerance significantly improved after balsamic vinegar treatment compared to the tap water treatment. In both the CD and HFD groups, the insulin area was significantly higher in the balsamic vinegar administered rats compared to water administrated rats. This difference suggests that balsamic vinegar can compensate for hypercholesterolemia-induced hyperglycemia by increasing insulin secretion.
Cholesterol is an essential component of many putative domains, however, excessive cellular cholesterol plays a direct role in pancreatic islet dysfunction and is a key factor underlining the progression of type 2 diabetes [10]. The mechanism of β-cell failure in type 2 diabetes is diverse and complicated. Associated genetic and acquired defects include oxidative stress, endoplasmic reticulum stress, and dysfunctional triglyceride/free fatty acid cycling [11]. Cholesterol imbalance, accompanied by a reduction in insulin secretion, plays a critical role in the pathophysiology of type 2 diabetes. Cholesterol accumulation in β-cells causes significant islet cell dysfunction [12]. Many previous studies have demonstrated that elevated islet cholesterol causes impaired insulin secretion [13,14]. Also, a recent study showed that ABCA1 is a key protein for cholesterol homeostasis in pancreatic β-cells [2,5]. In our study, pancreatic sections from balsamic vinegar-administered rats exhibited an increase in islet ABCA1, both in the CD group and the HFD group. Based on this finding, HFD induced hyperglycemia might be restored by balsamic vinegar treatment through increasing expression of β-cell ABCA1.
The primary limitations of this study were that exact quantification of islet cell cholesterol levels and ABCA1 levels were not available. Another limitation of this study is that we did not consider other cholesterol pathways that could have influenced β-cell insulin secretion.
In conclusion, our results suggest the possibility that balsamic vinegar could enhance β-cell ABCA1 expression and increase β-cell insulin secretion. Further studies are warranted to confirm the relationship between balsamic vinegar and ABCA1 activation.
ACKNOWLEDGMENTS
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean government (MOST) (No. R13-2002-054-04002-0).
Notes
No potential conflict of interest relevant to this article was reported.