Prevalence and Clinical Characteristics of Recently Diagnosed Type 2 Diabetes Patients with Positive Anti-Glutamic Acid Decarboxylase Antibody
Article information
Abstract
Background
Latent autoimmune diabetes in adults (LADA) refers to a specific type of diabetes characterized by adult onset, presence of islet auto-antibodies, insulin independence at the time of diagnosis, and rapid decline in β-cell function. The prevalence of LADA among patients with type 2 diabetes varies from 2% to 20% according to the study population. Since most studies on the prevalence of LADA performed in Korea were conducted in patients who had been tested for anti-glutamic acid decarboxylase antibody (GADAb), a selection bias could not be excluded. In this study, we examined the prevalence and clinical characteristics of LADA among adult patients recently diagnosed with type 2 diabetes.
Methods
We included 462 patients who were diagnosed with type 2 diabetes within 5 years from the time this study was performed. We measured GADAb, fasting insulin level, fasting C-peptide level, fasting plasma glucose level, HbA1c, and serum lipid profiles and collected data on clinical characteristics.
Results
The prevalence of LADA was 4.3% (20/462) among adult patients with newly diagnosed type 2 diabetes. Compared with the GADAb-negative patients, the GADAb-positive patients had lower fasting C-peptide levels (1.2±0.8 ng/mL vs. 2.0±1.2 ng/mL, P=0.004). Other metabolic features were not significantly different between the two groups.
Conclusion
The prevalence of LADA is 4.3% among Korean adult patients with recently diagnosed type 2 diabetes. The Korean LADA patients exhibited decreased insulin secretory capacity as reflected by lower C-peptide levels.
INTRODUCTION
Type 1A diabetes mellitus is caused by the autoimmune destruction of pancreatic β-cells, which leads to insulin deficiency resulting in a need for insulin therapy for most patients. Pancreatic auto-antibodies, such as islet cell cytoplasmic antibody (ICA) [1], glutamic acid decarboxylase antibody (GADAb) [2], and antibodies to the protein tyrosine phosphatases IA-2 or IA-2β, are used to diagnose type 1A diabetes [3]. However, as in typical cases of type 1A diabetes, some patients clinically diagnosed with type 2 diabetes have circulating auto-antibodies to pancreatic β-cells [4-7]. Type 2 diabetes with positive auto-antibodies is characterized by insulin independence at the time of diagnosis, a high incidence of relative insulin deficiency and rapid progression to insulin dependence [5-9]. This form of diabetes has also been called latent autoimmune diabetes in adults (LADA) [8], slowly progressive insulin-dependent diabetes mellitus [10] or type 1.5 diabetes [11]. LADA has been included in the proposal for the World Health Organization (WHO) criteria for diabetes as a subgroup of type 1 diabetes [12].
In the United Kingdom Prospective Diabetes Study (UKPDS), 10% of patients were positive for GADAb, and 52% of patients with a titer of GADAb ≥20 U/L required insulin treatment within 6 years after diagnosis [7]. Fourlanos et al. [13] found that LADA was commonly associated with the following clinical features: age of onset <50 years, acute onset of symptoms, body mass index (BMI) <25 kg/m2, and personal history or family history of autoimmune diseases.
There is a controversy about whether LADA can be viewed as a separate form of diabetes or rather a subgroup of type 1 diabetes [14]. Patients with LADA have a similar disease process as patients with type 1 diabetes in that they have a higher rate of progression to insulin dependency and a lower prevalence of metabolic syndrome [15,16]. However, LADA exhibits a slower progression toward insulin dependency compared to typical type 1 diabetes and also shows clinical features of insulin resistance. Recent studies reported that LADA shares genetic features of both type 1 and type 2 diabetes [17,18]. Therefore, LADA is considered a specific form of diabetes that lies somewhere between type 1 and type 2 diabetes [18].
In Korea, several studies have examined the prevalence and clinical characteristics of LADA [19-24]. However, the number of patients in most of these studies was small, and the study patients were tested for GADAb probably due to suspicion of a diagnosis other than type 2 diabetes. Thus, these studies had a limitation to represent the prevalence or clinical characteristics of LADA in Koreans. In this study, we measured GADAb in patients with recent onset type 2 diabetes and evaluated the prevalence of GADAb positivity and compared the clinical characteristics of patients with and without GADAb.
METHODS
Participants
This study was approved by the Institutional Review Board of Seoul National University Hospital (H-1106-127-369). Between January 2005 and July 2007, we tested GADAb in 749 patients over 20 years of age who first visited the Diabetes Clinic of Seoul National University Hospital. We selected 527 patients who were diagnosed with diabetes within the past 5 years and excluded patients with type 1 diabetes, those who started insulin therapy within 1 year after diagnosis of diabetes, and patients with a history of diabetic ketoacidosis. We also excluded patients who were pregnant, had chronic liver disease, acute infection, history of organ transplantation, current chemotherapy for malignancy, or other conditions that could affect glucose metabolism. Therefore, a total of 462 patients were included in the analysis.
Methods
GADAb test
GADAb was measured by a radioimmunoassay employing 125I-labeled human recombinant GAD65 manufactured by RSR (Cardiff, Wales, UK). GADAb values >1.0 U/mL were considered positive.
Laboratory methods
Biochemical tests (such as serum levels of glucose and lipids) were measured with the Toshiba 200FR Neo Chemistry autoanalyzer (Toshiba Medical Systems Co., Tokyo, Japan) after overnight fasting. Hemoglobin A1c (HbA1c) was determined by an affinity chromatography (Bio-Rad Laboratories, Hercules, CA, USA), and the C-peptide level was evaluated with a radioimmunoassay (TFB, Tokyo, Japan). The serum insulin concentration was measured using a immunoradiometric assay (Biosource Europe S.A., Nivelles, Belgium). Insulin resistance and β-cell function were estimated using homeostasis model assessment (HOMA-IR and HOMA-β, respectively) [25].
Physical examination
We measured height, weight, and blood pressure with standard methods. The waist circumference across the area midway between the lowest rib margin and the highest point of the iliac crest and the hip circumference across the widest level over both the greater trochanters were measured.
Statistical analysis
Statistical analyses were performed using the SPSS (SPSS Inc., Chicago, IL, USA). Data are given as the mean±standard deviation. χ2-test, Fisher's exact test, Student's t-test, and Mann-Whitney U test were used where appropriate. A two-tailed P value less than 0.05 was considered statistically significant.
RESULTS
Prevalence of GADAb positivity
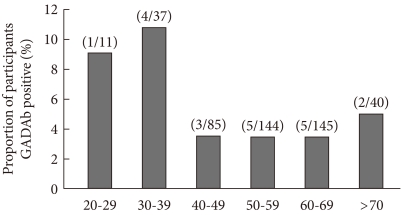
Of 462 patients, 20 (4.3%) were positive for GADAb. The GADAb positivity among men and women was 4.6% (12/260) and 4.0% (8/202), respectively. GADAb was found in 9% to 10% of patients in their 20s or 30s, less than 4% in patients in their 40s, 50s or 60s, and in 5% of patients over 70 (Fig. 1).
Clinical characteristics of type 2 diabetic patients according to presence of GADAb
The mean ages of the patients with positive GADAb (n=20) and negative GADAb (n=442) were 52.3±14.1 and 55.3±11.6, respectively. There was no difference in age, age at the time of diagnosis of diabetes, or the duration of diabetes between GADAb-positive and GADAb-negative patients. Proportions of patients taking antihypertensive medication or 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor were not different in the groups. Three of 20 (15.0%) GADAb-positive patients required insulin treatment compared to 24 of 442 (5.4%) GADAb-negative patients, a difference which was only marginally significant (P=0.074).
BMI values were not statistically different between the two groups (23.7±3.6 kg/m2 vs. 25.2±3.6 kg/m2, P=0.101), although BMI tended to be lower in patients with positive GADAb. C-peptide levels were significantly lower in GADAb-positive patients than in GADAb-negative patients (1.2±0.8 ng/mL vs. 2.0±1.2 ng/mL, P=0.004). The two groups did not differ with respect to waist circumference, waist-to-hip circumference ratio, systolic blood pressure, diastolic blood pressure, fasting insulin levels, HbA1c, fasting glucose levels, or serum lipid profile (including total cholesterol, triglyceride, high density lipoprotein cholesterol [HDL-C], and low density lipoprotein cholesterol [LDL-C]). GADAb-positive and GADAb-negative patients had similar values of HOMA-insulin resistance (IR) and HOMA β-cell function (Table 1).
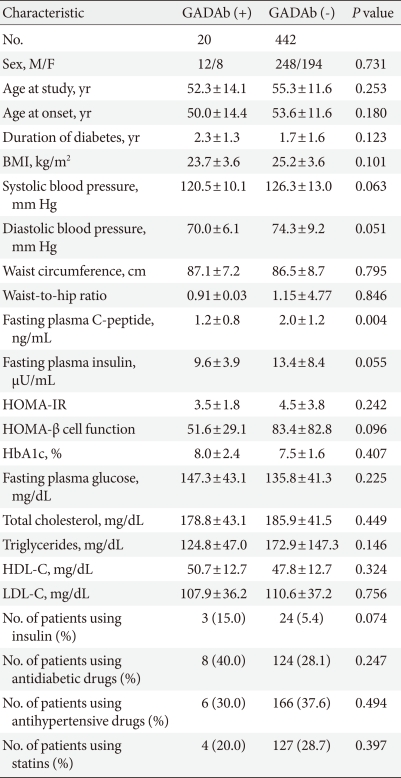
Clinical characteristics of recently diagnosed type 2 diabetic patients according to the presence or absence of GADAb
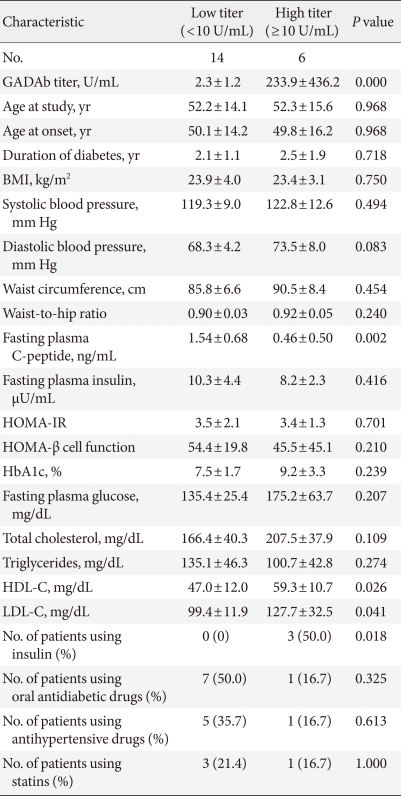
According to the titer of GADAb, we divided the LADA patients into high- (≥10 U/mL) and low-titer (<10 U/mL) subgroups, which was a similar analytic approach as that used in a previous study [26]. C-peptide levels were higher in LADA patients with a low-titer of GADAb than in those with a high-titer of GADAb. The HDL-C and LDL-C concentrations were higher in the LADA patients with a high titer of GADAb than in those with a low titer of GADAb. The LADA patients with a high titer of GADAb were more likely to require insulin than those with a low titer of GADAb (Table 2).
DISCUSSION
Type 1A diabetes mellitus results from the autoimmune destruction of insulin-producing β-cells in pancreatic islets. ICA and GADAb are markers of autoimmunity and are detected in 70% to 80% of patients with type 1 diabetes [27]. LADA is defined as the initially non-insulin-requiring condition of diabetes with autoimmune markers of type 1 diabetes, such as ICA and GADAb. Progressive β-cell destruction by autoimmune mechanisms is considered as the main pathogenesis of LADA [28,29]. IA-2 antibody is detected in a high frequency at diagnosis in type 1 diabetic children, whereas the frequency is lower in LADA patients [30]. In addition, GADAb has a higher sensitivity compared with ICA [9,17]. Therefore, we used GADAb to identify cases with LADA in this study.
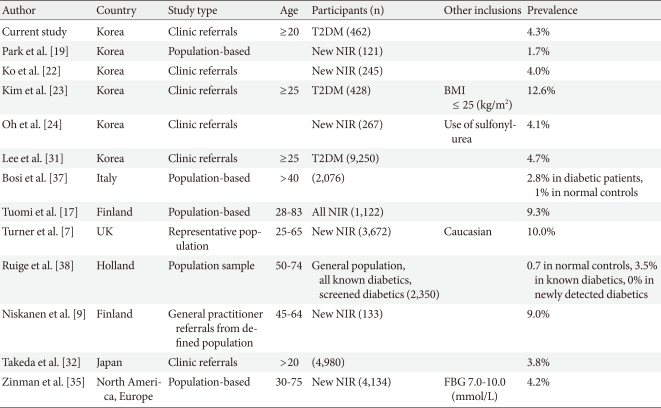
In previous studies performed in Korea, the prevalence of GADAb-positivity in type 2 diabetes was reported to be 1.7% to 12.6% [19-24]. In the current study, 4.3% of patients were positive for GADAb, which represents similar findings as those reported by Ko et al. [22], Oh et al. [24], and Lee et al. [31]. Most previous Korean studies selected patients who were non-obese or patients tested for GADAb who were under clinical suspicion of a diagnosis other than type 2 diabetes. In our study, we tested GADAb in all type 2 diabetic patients with a recent onset (within the past 5 years) regardless of their clinical characteristics at the time of presentation, which might help us avoid selection bias and therefore more accurately estimate the unbiased prevalence of LADA.
The prevalence of GADAb-positivity in this study was similar to the prevalence in Japanese studies [32,33]; however, it was lower than that reported in China [34] or Western countries [7,17]. The prevalence of LADA has been estimated in a number of studies (including large-scale studies) [7,35], revealing a wide range of variations from 2% to 20% (Table 3). The prevalence of LADA has been shown to vary even within the same country. In Swedish studies, the frequency of LADA ranged from 8% to 24%. A wide range of variation in the prevalence of LADA within a country or across countries may depend on criteria for diagnosis, antibody assay methods, characteristics of the patients, and genetic susceptibility.
In previous studies of Western countries, LADA patients have a lower BMI, more decreased insulin secretory capacity at diagnosis, and more rapid progression to insulin dependence than typical type 2 diabetes patients [7,9,17]. In the current study, the BMI of the patients with positive GADAb tended to be lower than that of the patients with negative GADAb, which was not a statistically significant difference. In addition, components of metabolic syndrome, such as hypertension, hypercholesterolemia, abdominal obesity, and fasting hyperglycemia, were evaluated in this study. No difference was seen between GADAb-positive and GADAb-negative patients with respect to the use of antihypertensive medication or HMG-CoA reductase inhibitors; serum levels of fasting glucose, triglycerides, total cholesterol, LDL-C, and HDL-C; or waist circumference and waist-to-hip circumference ratio. Patients with GADAb tended to have higher systolic and diastolic blood pressure than patients without GADAb. However, these differences were not statistically significant. These results were contrary to the findings of other studies. Tuomi et al. [17] reported that patients with GADAb had lower blood pressure, a lower level of triglycerides, and a lower waist-to-hip circumference ratio compared with patients without GADAb. The ADOPT study revealed that patients with positive GADAb had a higher level of serum HDL-C, lower serum triglyceride levels, and a lower prevalence of metabolic syndrome than patients with negative GADAb [35]. In another study performed in Caucasian populations, metabolic syndrome was more frequent in type 2 diabetes patients without GADAb than it was in LADA patients [16]. The mean BMI of our study patients was 25.3 kg/m2, which is lower than that of Western studies (27.5 to 32 kg/m2) [7,17,35] and may explain different features related to metabolic syndrome among different ethnic groups. In this study, however, many patients without GADAb were treated with HMG-CoA reductase inhibitors, which might have contributed to attenuating the difference in lipid profiles according to the presence of GADAb. Nonetheless, even though there was no difference in the use of HMG-CoA reductase inhibitors between high- and low-GADAb titer groups, the HDL-C and LDL-C concentrations were lower in LADA patients with a low titer of GADAb than they were in those with high GADAb titers. The apparent absence of an association between LADA and the features of metabolic syndrome may be due to the small number of participants in our study. Therefore, a large-scale, nationwide study is warranted to examine the clinical characteristics related to metabolic syndrome in patients with LADA in Korea.
We observed that GADAb-positive patients had lower C-peptide levels than GADAb-negative patients, which suggests that β-cell destruction by autoimmunity may elicit decreased insulin secretory capacity in patients with LADA [31]. In addition, patients who were positive for GADAb tended to use insulin more frequently than patients who were negative for GADAb. Furthermore, the LADA patients with a high titer of GADAb obviously needed more insulin therapy and had lower C-peptide levels than the LADA patients with a low titer of GADAb, which was consistent with the results of a previous study [17]. Because patients with LADA also exhibit insulin resistance like that in type 2 diabetic patients, they may require a different therapeutic approach than the usual type 2 diabetic patients. Sulfonylureas are generally not recommended as the first-line therapy for LADA because these drugs may bring about earlier insulin dependence [36]. As was shown in the present study, the clinical characteristics of LADA in Korea may be different from those in Western countries; it may be necessary to develop a therapeutic strategy unique to Korean patients.
In conclusion, we showed that the prevalence of LADA was 4.3% among recently diagnosed type 2 diabetic patients in Korea. The Korean LADA patients exhibited decreased insulin secretory capacity in terms of C-peptide level. Considering the unique pathogenetic mechanism of LADA, encompassing not only prominent insulin deficiency but also insulin resistance, it is mandatory to develop an effective therapy to reduce hyperglycemia and to halt the progressive beta-cell failure in these patients.
ACKNOWLEDGMENTS
This study was supported by Grant 00-PJ3-PG6-GN07-001 from the Genome Research Center for Diabetes and Endocrine Disease, Ministry of Health & Welfare, Republic of Korea.
Notes
No potential conflict of interest relevant to this article was reported.