- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 48(1); 2024 > Article
-
Original ArticleBasic Research A New Concept in Antidiabetic Therapeutics: A Concerted Removal of Labile Iron and Intracellular Deposition of Zinc
-
Vladimir Vinokur1,2
 , Eduard Berenshtein1, Mordechai Chevion1, Dror Chevion2
, Eduard Berenshtein1, Mordechai Chevion1, Dror Chevion2
-
Diabetes & Metabolism Journal 2024;48(1):59-71.
DOI: https://doi.org/10.4093/dmj.2022.0292
Published online: January 3, 2024
- 1,676 Views
- 178 Download
1Department of Biochemistry and Molecular Biology, Institute of Medical Research Israel-Canada, The Hebrew University of Jerusalem (HUJI), Jerusalem, Israel
2Concenter Biopharma, Jerusalem, Israel
-
Corresponding author: Dror Chevion
 Department of Biochemistry and Molecular Biology, Institute of Medical Research IsraelCanada, The Hebrew University of Jerusalem, 18 Ha-Yahalom, Tel Mond 4061818, Jerusalem, Israel E-mail: dror@concenterbiopharma.com
Department of Biochemistry and Molecular Biology, Institute of Medical Research IsraelCanada, The Hebrew University of Jerusalem, 18 Ha-Yahalom, Tel Mond 4061818, Jerusalem, Israel E-mail: dror@concenterbiopharma.com
Copyright © 2024 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
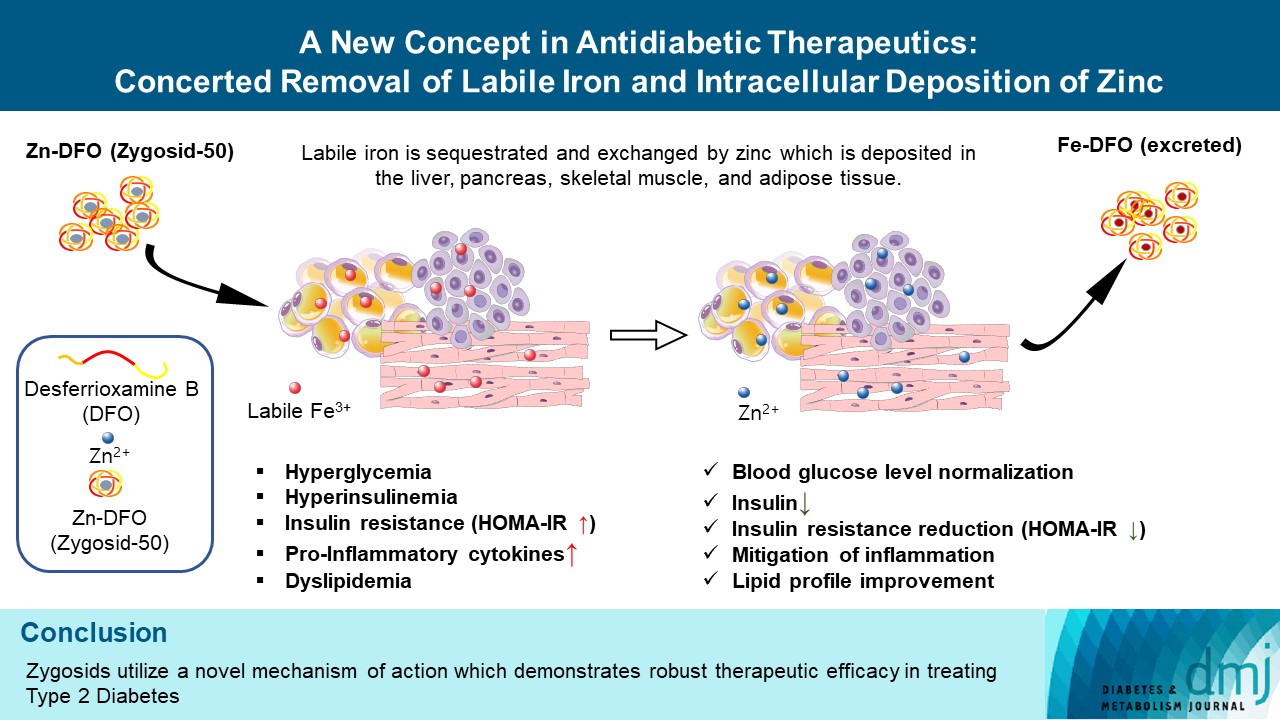
- The inflammatory process is known to be an integral part of the pathophysiology of type 2 diabetes mellitus (T2DM). The “labile,” redox-active iron, serving as a catalyst in Fenton reaction, producing the deleterious reactive oxygen species, triggering and maintaining inflammation, is hypothesized to play a causative role in this process. Concenter Biopharma continued the development of a new platform of iron chelators (Zygosids), first initiated at the Hebrew University of Jerusalem, Israel (HUJI), acting via the novel mechanism, based on a sequestration of the labile redox-active iron and its substitution by zinc or gallium. The mode of action of Zygosids is based on the higher affinity of the metal-binding moiety of the complex to Fe3+ in comparison to already bound ion, leading to rapid release of the ion of another metal and chelation of Fe3+. Concomitantly, zinc ion, released by the complex, is known for its antidiabetic and anti-inflammatory role.
-
Methods
- The therapeutic effect of zinc-desferrioxamine (Zygosid-50) and gallium-desferrioxamine, was tested on fat sand rat (Psammomys obesus) model of diet-induced T2DM and on Leprdb transgenic diabetic mice.
-
Results
- Zygosids demonstrated an ability to noticeably reduce blood glucose and insulin levels and improve the lipid profile. Moreover, an ability to mitigate insulin resistance by >90% was shown on the sand rat model. In addition, a potent anti-inflammatory effect, expressed as a diminishment of the proinflammatory cytokines in tissue levels, was demonstrated.
-
Conclusion
- Zygosids demonstrated robust therapeutic efficacy in treatment of T2DM. Importantly, no adverse effects were detected, in all the experiments, indicating high safety profile.
- Type 2 diabetes mellitus (T2DM) is a common illness. Currently, an estimated 37.3 million people in the United States have contracted T2DM, and another 84.1 million have prediabetes, a condition that if not treated, often leads to T2DM within 5 years. The incidence of T2DM is increasing because of more advanced aging of populations, sedentary lifestyle, diet, and the alarming increase in the prevalence of obesity. It is expected that the prevalence of diabetes will nearly double by year 2050. The annual cost of T2DM in 2017 in the United States alone was estimated at US $402.3B [1]. Also, the T2DM share across the major markets (USA, France, Germany, Italy, Spain, the United Kingdom, and Japan), is expected to double in value from 2016 to 2026 [2].
- The attempts to treat T2DM and its complications are being constantly made. Nevertheless, complications are still common, and T2DM is among the leading causes of death, vision loss, amputation, and end-stage renal disease, in the USA.
- It is highly important to notice that although numerous antidiabetic medications are available on the market now, all of them are targeted at symptoms of the disease, without almost no influence on the high and constantly rising levels of insulin resistance (IR), the core feature of the disease. Moreover, the use of medications is often accompanied by severe adverse side effects, including the risk of life-threatening hypoglycemia.
- A line of publications have disclosed incriminating evidence, demonstrating a causal relationship between the accumulation of labile redox-active iron within various tissues, including pancreatic cells, hepatocytes, and peripheral adipose tissues, and T2DM development [3]. Iron affects glucose metabolism, which in turn, impinges on iron metabolic pathways, creating vicious cycle. Briefly, accumulation of labile redox-active iron within various tissues contributes to formation of reactive oxygen species (ROS) via Fenton reaction. ROS promote the initiation of an inflammatory response, which in turn, underlies IR and metabolic syndrome [4], playing a pivotal role in the pathophysiology of T2DM [5]. Still, iron was suggested to be functionally important for normal insulin secretion and pancreatic β-cells metabolism. Exposure of pancreatic islets to high glucose concentrations stimulates production of ferritin, serving as a mechanism for the β-cells to facilitate and maintain the critical iron stores during insulin secretion. Accordingly, hepcidin, iron-regulating hormone, produced by liver and pancreas to facilitate intracellular iron accumulation by binding the iron exporter ferroportin (Fpn) and mediating its internalization and degradation [6], is located within the insulin secretory vesicles islets and secreted after stimulation with high glucose [7]. Since Fpn is expressed by β-cells, hepcidin thus exerts an autocrine inhibitory action on β-cells iron export [7].
- In liver, excessively accumulated iron interferes with glucose metabolism via both decreased insulin clearance and impaired insulin signaling. These phenomena result in hyperinsulinemia, which, in turn, promotes the intra-hepatic iron buildup [8]. Insulin was reported to directly enhance the uptake of extracellular iron, inducing the redistribution of transferrin receptor on the cell surface [9]. Thus, it was hypothesized that “iron and insulin are synergistic in promoting oxidative stress with release of ROS and inflammatory cytokines” [10]. These cytokines promote ferritin synthesis in Kupffer cells and macrophages [3]. In adipose tissues iron accumulation was associated with visceral adipose tissue hyperplasia/hypertrophy, followed by impaired insulin sensitivity, macrophages infiltration and proinflammatory cytokines production [11].
- Increased iron stores were shown to predict the development of T2DM, while iron depletion was protective [12]. Intracellular labile redox-active iron often induces damage and modulates the development of T2DM complications, including nephropathy, retinopathy, cardiomyopathy, and peripheral neuropathy [12].
- Attempts have been made to treat diabetes with several iron chelators, including Desferal (where the active component is desferrioxamine [DFO]-B; Novartis, Basel, Switzerland), yielding controversial results that did not justify its use as an antiT2DM drug [13,14]. Although iron-chelating medications demonstrated certain efficacy against T2DM, caused by primary or secondary hemochromatosis [15], in other subjects the success was limited.
- The main drawback of Desferal is that its iron-binding component (DFO) does not penetrate membranes and, therefore, cannot scavenge the intracellular labile iron. DFO is a long-linear polar molecule; this feature explains its lack of infiltrability into cells. Binding a zinc ion to DFO and forming the newly developed zinc (Zn)-DFO complex (for the commercial purposes called Zygosid-50), renders the molecule less polar, having a relatively tight globular structure, which readily passes through cellular membranes. Within the cells, the zinc ion of the Zn-DFO complex readily exchanges with cellular labile iron. The process was suggested by Chevion [16] in 1991 and the term “push-pull reaction” was coined. Thus, the main obstacle of iron chelation by Desferal can be overcome using the Zn-DFO complex [16], so that intracellular toxic iron is sequestrated and removed. This exchange reaction is explained by the affinity constants of DFO to Fe3+ [17], which is markedly higher than that of DFO to zinc (stability constants are 1031 and 1011, respectively) [17]. When Zn-DFO enters the cell, in the presence of intracellular labile iron, a rapid exchange of the zinc by iron occurs, forming the Fe-DFO complex. Zinc ions remain within the cell, and the Fe-DFO complex is mobilized to the circulation system and subsequently removed from the body [16].
- Zinc ions by themselves were shown to play important roles in β-cell function (insulin synthesis, its packing/storage and secretion), in glucose homeostasis and, per contra, in the pathogenesis of T2DM and its complications. Normal β-cells contain large amounts of zinc, where one of its major functions is the binding and packaging of insulin in a hexameric form with two zinc ions, required for long-term stability of this protein [18]. In addition, zinc ions, released together with insulin, are involved in paracrine regulation of α- and β-cells and glucagon secretion [19]. A line of studies has demonstrated that zinc has insulin-mimetic properties [20], modulating insulin signaling pathways, and enhancing glucose uptake by cells and its clearance from the blood [21]. Additional important roles of zinc have been identified in the regulation of lipids metabolism, lipogenesis, and adipose tissue inflammation, directly related to T2DM [22]. Accordingly, zinc deficiency is often observed among T2DM patients and, more generally, is associated with β-cell malfunctioning, IR, and severe atherosclerosis [23].
- Thus, the purpose of this study was to test the antidiabetic efficacy of Zygosids, the novel platform of drugs acting via the mechanism, involving sequestration of the redox-active iron and its substitution by zinc, known to have an antidiabetic activity, or gallium.
INTRODUCTION
- The studies were performed on two T2DM models. All animals, if other not stated, were purchased from the Authority for Biological and Biomedical Models, The Hebrew University of Jerusalem, Israel, and the experimental protocols (MD-08-11765-3 and MD-13-13922-3) were approved by it.
- The first: The sand rat (Psammomys obesus) is known to develop diabetes spontaneously, when fed a high-energy diet (HED), mimicking the nutritionally-induced T2DM in human, with elevated IR and progressive β-cell failure [24]. Once hyperglycemia develops (blood glucose level [BGL] >200 mg/dL), a rapid deterioration to T2DM occurs, reaching the endstage of the disease, characterized by severe hyperlipidemia, and ketosis, within 4 to 6 weeks of commencement of the HED. Continued dietary load superimposed on IR results in depletion of pancreatic insulin stores, with increased proportions of insulin precursor molecules in the pancreas and blood. The major culprit is inappropriate insulin production, with insulin stores depletion as a consequence. Similar mechanisms operate during the evolution of T2DM in humans. Hyperglycemic sand rats tend to develop microangiopathic complications, resulting in diabetic nephropathy and diabetes-induced cataract.
- The second animal model: Mice, homozygous for the diabetes spontaneous mutation (leptin receptor [Lepr]db), manifest morbid obesity, chronic hyperglycemia and pancreatic β-cell atrophy. The severity of disease leads to uncontrolled blood sugar rise, severe depletion of insulin-producing pancreatic β-cells, peripheral neuropathy, myocardial disease and death by 10 months of age. Exogenous insulin fails to control BGLs and gluconeogenic activity increases.
- Zygosids were prepared as described in Bibi et al. [25] and administered as an aqueous solution. The sand rats’ studies included the prophylactic and therapeutic studies.
- Prophylactic study on sand rats
- In the first set of experiments 55 10- to 12-week-old male sand rats from the diabetes-prone sub-strain were fed the HED from day 1. The animals were divided into six groups: Group 1: low energy diet, untreated controls (n=12); Group 2: HED, sham-treated (n=10); Group 3: HED, treated with Zygosid-50 2 mg/kg (n=8); Group 4: HED, treated with Zygosid-50 6 mg/kg (n=9); Group 5: HED, treated with gallium-DFO (Ga-DFO), 2 mg/kg (n=8); Group 6: HED, treated with Ga-DFO complex, 6 mg/kg (n=8).
- Zygosids were given by intraperitoneal (i.p.) injection 3×/week, from day 1 of the study. Throughout the experiment the animals’ body weight was monitored 2/week, and blood glucose 3/week. On day 52 the animals were euthanized, and blood was taken for assessment of lipid profile (total serum cholesterol, high-density lipoprotein [HDL], low-density lipoprotein [LDL], triglycerides) and serum insulin. Epididymal fat as a percentage of the total body weight was assessed as well.
- Therapeutic study on sand rats
- In the second set of experiments 52 10- to 12-week-old male from the diabetes-prone sub-strain were divided into five groups: Group 1: low energy diet, untreated (n=8); Group 2: HED, sham-treated (n=14); Group 3: HED, treated with Zygosid-50 2 mg/kg (n=10); Group 4: HED, treated with Zygosid-50 6 mg/kg (n=12); Group 5: HED, treated with Ga-DFO, 6 mg/kg (n=10). Treatment was started on day 27/28, only after the animals had already contracted diabetes (BGL ≥250 mg/dL), having BGL=286±13 mg/dL, by day 21–23 of the study. To assess the effect of Zygosids on IR, glucose tolerance test (i.p.-GTT, 1 g glucose/kg) was performed on the day 57, toward the end of the experiment, as described by Anis et al. [26]. Throughout the experiment the body weight was monitored 2×/week and BGL 3×/week. On the last day of the experiment cataract formation was visually assessed and scored. After euthanasia, the same parameters as described in the prophylactic experiment (above), and blood markers of non-alcoholic steatohepatitis (NASH) (aspartate transaminase [AST]/alanine transaminase [ALT]) were measured. Livers were excised and stored in paraformaldehyde 4% for histological studies. Samples from the liver underwent H&E staining and analyzed for NASH severity score (Brunt et al. [27], measuring the percent of intra-hepatocyte macro-vesicular fat: 0%, score 0; <30%, score 1; 31%–50%, score 2; >51%, score 3).
- Therapeutic study on Leprdbmice
- The third set of experiments was performed on Leprdb mouse mutants. Eight male BKS.Cg-Dock7m+/+ Leprdb/J mice 8 to 9 weeks old were purchased and imported from Jackson Labs (Bar Harbor ME, USA). The animals were divided into two groups of n=4. Group 1 was treated with Zygosid-50 6 mg/kg body weight thrice a week for 60 days, while the second served as a sham-treated control. Body weight and BGL were monitored as described above. i.p.-GTT (2 g glucose/kg) was performed in the beginning of the experiment and 2 days before the euthanasia. Serum level of insulin was assessed before the first treatment and on the last day.
- Anti-inflammatory activity
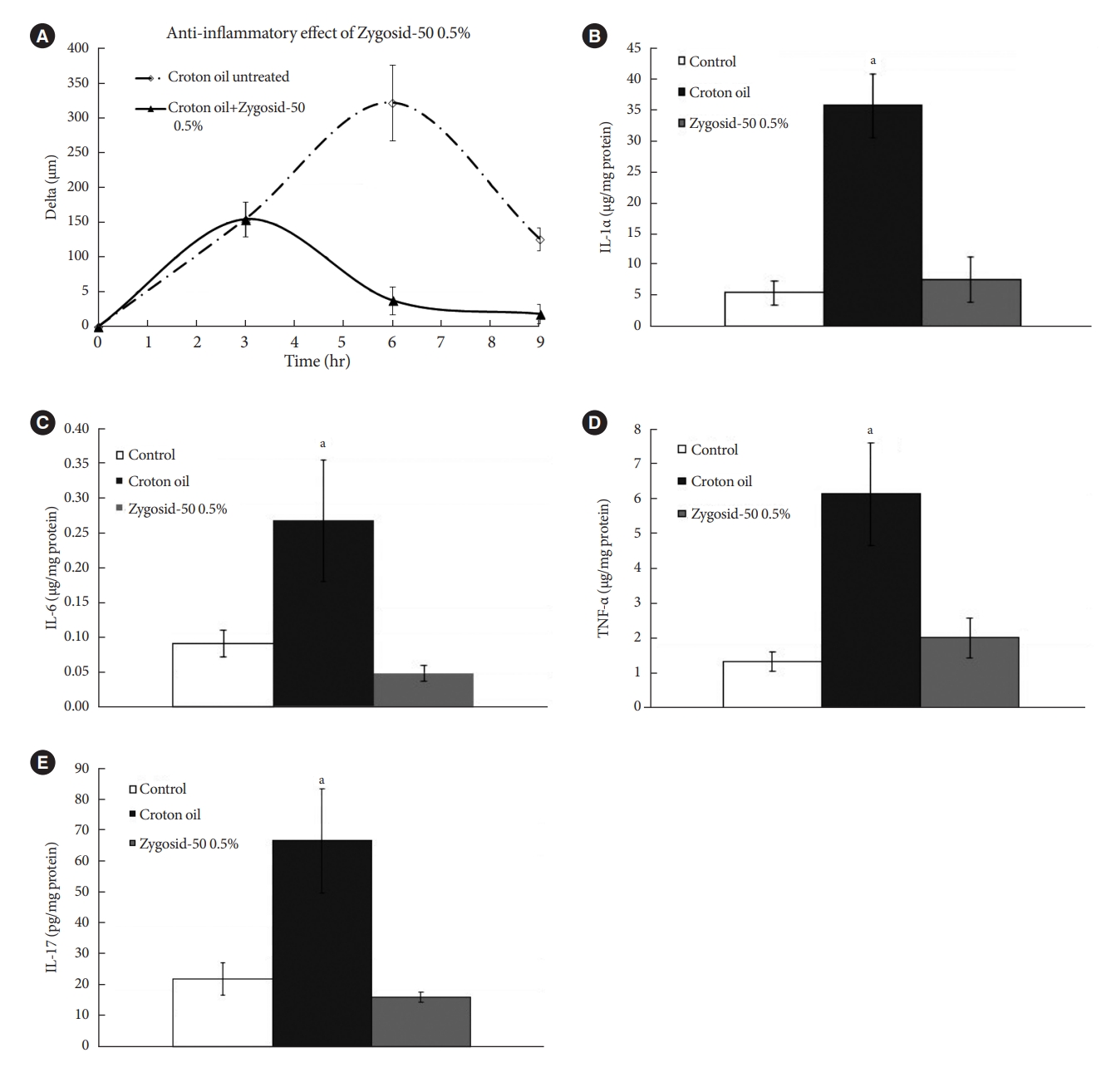
- In order to elucidate the mode of Zygosid-50 anti-inflammatory action and to assess its ability to suppress production of proinflammatory cytokines, associated with T2DM, an additional assay was performed using the croton oil-induced model of skin inflammation [28]. Three groups of 12-week-old female Balb/c mice (n=6 per group) were used. Irritant contact dermatitis was induced by epicutaneous application of 15 µL 1% croton oil in acetone and olive oil (4:1) to the right ear. Ear thickness was monitored with an engineer’s micrometer, along the experiment: immediately before the exposure to the croton oil, and at 3, 6, 9 hours following the exposure. The pre-exposure thickness value was subtracted from the post-exposure values at each time point, quantifying the severity of inflammation.
- Group 1 remained untreated. A fingertip of Zygosid-50 0.5% ointment was applied once (Group 2), 3 hours after the first exposure to the irritant. Six hours after the exposure the mice were euthanized, the right ears were immediately harvested and placed in liquid nitrogen. The samples were subsequently homogenized. Cytokine concentrations were determined by enzyme-linked immunosorbent assay (ELISA), employing commercially available kits. The cytokines measured included: interleukin 1α (IL-1α), IL-6, tumor necrosis factor-α (TNF-α), and IL-17.
- Statistical analysis
- The results are reported as the mean±standard error. For all experiments, sample size was defined based on the resource equation method, taking into consideration a possible death rate of 10%, where applicable. The animals were dispatched between the cages in a random queue order (V.V., E.B.). The confounders were not controlled. Blinding took place at the stage of the data analysis only (V.V., E.B.).
- The data was analyzed using repeated one-way analysis of variance (ANOVA) followed by the Scheffé post hoc test for multiple comparisons (α=0.05) (Microsoft Excel). Statistically significant differences were considered when P≤0.05.
METHODS
- Prophylactic administration of Zygosids: sand rats
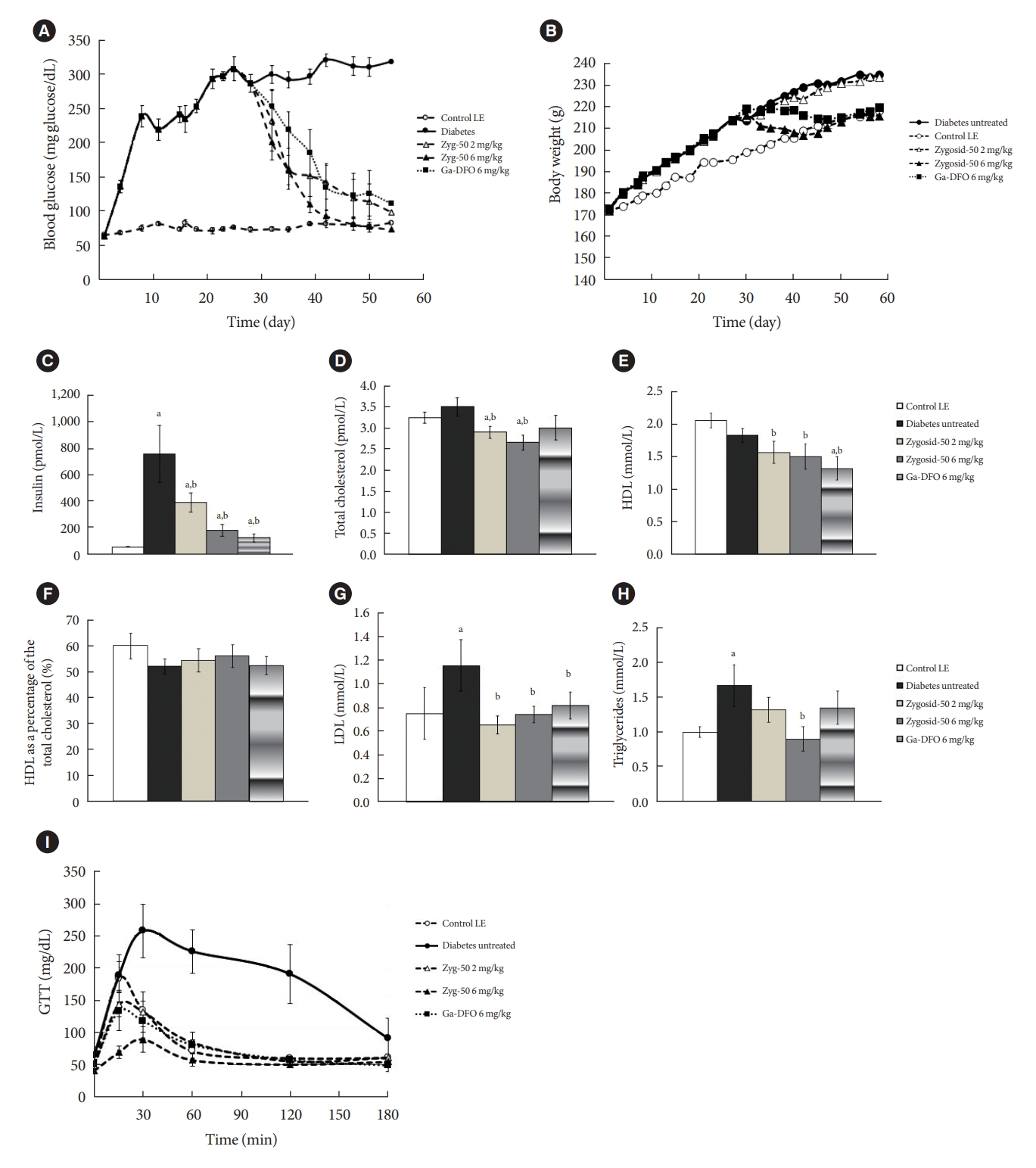
- Feeding sand rats HED resulted in a rapid increase in BGL, reaching >300 mg/dL after 2 to 3 weeks. Among the animals fed low-energy diet (LED), BGL remained normal and stable. Prophylactic treatment of the HED-fed animals with Zygosid-50 2 and 6 mg/kg 3×/week, inhibited the process of T2DM development in a dose-responsive manner. The Ga-DFO complex demonstrated even higher prophylactic efficacy (Fig. 1A). The rate of weight gain of untreated animals, fed HED was markedly higher than that of the untreated LED. Administration of either Zygosid (6 mg/kg) suppressed the weight gain to the same extent (Fig. 1B). None of these treatments had an effect on the amount of epididymal fat-its percentage of total body weight did not vary, neither when fed HED, nor following any treatment described above (data not shown).
- Feeding on HED led to an increase in serum insulin level. Prophylactic administration of Zygosid-50 or Ga-DFO, at a dose of 2 mg/kg, 3×/week, reduced, partly, serum insulin level. Either Zygosid, at a dose of 6 mg/kg, 3×/week, markedly attenuated the development of hyper-insulinemia, keeping the level of serum insulin at near-normal range (Fig. 1C).
- The lipid profile of the sand rats, including total serum cholesterol, HDL, LDL, and triglycerides was also monitored. HED-fed animals demonstrated increased levels of total cholesterol. Treatment with Zygosid-50 or Ga-DFO, at either dose, prevented this increase, and maintained total serum cholesterol level, within the normal range, or even below. HDL fraction was slightly reduced by the HED, when compared to LED. Preventive treatment, when fed HED, with Zygosid-50 or Ga-DFO caused the HDL fraction to remain at the normal level. No significant changes in the concentration of LDL were observed after feeding on HED. Ga-DFO, in both doses, and Zygosid-50 at 6 mg/kg decreased the amount of LDL to below the basal level. Serum triglycerides level increased in the T2DM group. Neither Zygosid-50 or Ga-DFO were able to preserve serum triglycerides level within the normal range (Fig. 1C-H).
- The purpose of this experiment was to assess the prophylactic effect of Zygosids, administered concomitantly with exposure to diabetogenic diet. The outcomes show the efficacy and ability to prevent the development of T2DM during its early stages.
- Treatment of the diabetic sand rats with Zygosids
- The therapeutic effect of Zygosids against T2DM was assessed. All animals (except for the LED-fed control group) were fed HED. Their BGL increased rapidly. No animal was excluded from the study due to inability to develop the disease. Zygosid-50 or Ga-DFO led to the normalization of BGL (Fig. 2A). Zygosid-50 showed higher efficiency than Ga-DFO.
- Expectedly, HED-fed animals gained weight faster than LED-fed (Fig. 2B). Treatment with Zygosid-50 or Ga-DFO reduced the weight-gaining rate to that of sand rats fed on LED, attenuating the development of the T2DM-associated obesity. Zygosid-50 was more effective than Ga-DFO. Yet, the amount of epididymal fat remained unchanged, independently of the type of diet and the specific treatment the animals had experienced (data not shown).
- The hyperglycemia, observed in HED-fed animals, was associated with hyperinsulinemia. In LED-fed animals, blood insulin level remained within the normal range. Treatment with Zygosid-50, 2 mg/kg, 3×/week, reduced the level of serum insulin, but it was still slightly higher than normal. Administration of Zygosid-50 and Ga-DFO at doses of 6 mg/kg blood brought insulin values almost to the normal level (Fig. 2C).
- Further, the diabetogenic HED insignificantly increased the total amount of serum cholesterol compared to the control values. The treatment with Zygosid-50 or Ga-DFO reduced these values to slightly below normal. The HDL fraction of the total cholesterol in the hyperglycemic animals was lower than in the control. Treatment had no effect on this parameter. On the other hand, an increase in LDL concentration, though statistically non-significant, was observed in the diabetic animals. This increase was abolished in all treated groups. The HED resulted in increased levels of serum triglycerides. Treatment with Zygosid-50 or Ga-DFO alleviated this increase, although only the effect of Zygosid-50 6 mg/kg was statistically significant (Fig. 2D-H).
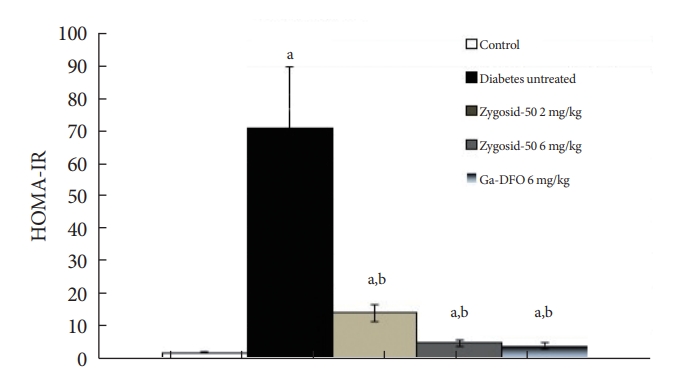
- Another accepted method to estimate IR is a calculation of homeostasis model assessment of insulin resistance (HOMA-IR) levels according to the following formula:
- Fasting glucose (mg/dL)×fasting insulin (µU/mL)/405.
- An impressively potent effect of Zygosid-50 or Ga-DFO on IR was observed. Zygosid-50 and Ga-DFO demonstrated an ability to diminish this parameter noticeably (Fig. 2I), while the administration of either one at doses of 6 mg/kg reduced IR almost to the normal level (Fig. 3).
- T2DM was shown to affect various organs, including eyes and liver. Analogously to humans, these complications have been reported for the diabetic sand rats [23]. Zygosid-50 and Ga-DFO demonstrated an impressive therapeutic effect against NASH, reducing blood ALT to the basal level (Fig. 4A). Consistently, NASH histological severity score of livers, obtained from the diabetic animals, treated with Zygosid-50 or Ga-DFO, was reduced vs. the sham group (Fig. 4B). No changes were observed in level of blood AST (data not shown). Furthermore, Zygosid-50 or Ga-DFO mitigated formation of diabetes-induced cataract (Fig. 4C and D). No signs of diabetic nephropathy were observed, so blood level of creatinine remained unchanged (data not shown).
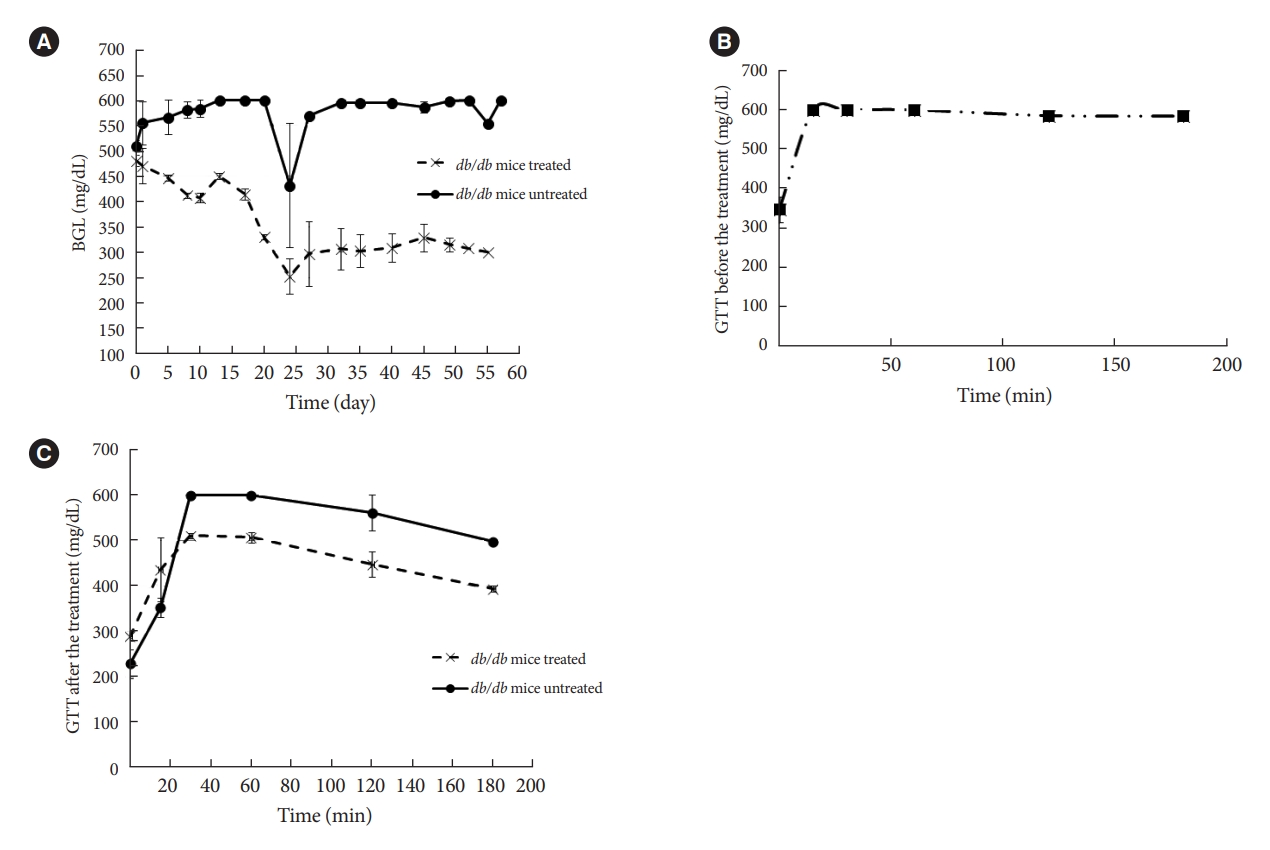
- Treatment of Leprdb mice
- Zygosid-50 noticeably reduced the severity of hyperglycemia in db/db mice (Fig. 5A), while no effect on body weight was detected (data not shown). An analogous trend was observed in glucose tolerance - which was improved to a certain extent, in comparison to the initial values (Fig. 5B), being still impaired (Fig. 5C). No statistically significant changes were observed in blood insulin levels (data not shown). Therefore, Zygosid-50 has shown an impressive effect against T2DM.
- Mode of the anti-inflammatory action
- Zygosid-50 0.5% ointment demonstrated an ability to mitigate the inflammation, induced by the croton oil (Fig. 6A). Exposure of mice ears to the irritant caused a marked (2.5- to 7.5-fold) increase in tissue levels of the cytokines. Zygosid-50 0.5% ointment abolished this effect, restoring the basal level of each cytokine (Fig. 6B-E). The hypothesized anti-T2DM effect of Zygosid-50 is based on activity in two aspects, i.e., the direct mitigation of IR, and suppression of the proinflammatory cytokines. This assay demonstrated Zygosid-50 potent anti-inflammatory activity.
RESULTS
- Investigating the extent of the antidiabetic activity of Zygosid-50, we presume that it is based on two synergistic actions, i.e., (1) chaperoning of zinc ions into the cell and their deposition within the intracellular milieu, conjunctly with (2) sequestration of the redox-active iron ions and their removal from the cell.
- Both processes, acting in concert in a strictly unidirectional manner, were expected, according to the initial hypothesis on the role of iron in T2DM, to have a profound antidiabetic and, more generally, an anti-inflammatory effect, alleviating IR suppressing production of proinflammatory cytokines and Improving glucose control.
- A causal relationship between accumulation of iron, within the pancreas itself, liver, and peripheral adipose tissue, and development of metabolic syndrome followed by subsequent T2DM has already been demonstrated in the literature [3,5]. Accumulation of labile redox-active iron contributes to the formation of ROS, which trigger inflammation and underlies IR [3,5]. According to one of the hypotheses, the process was suggested to be promoted by high rate of fatty acids mobilization from the peripheral adipose tissue due to iron-stimulated lipolysis [29]. Analogous phenomena of iron-stimulated fatty acids release, resulted in decreased response to insulin were observed in liver [30] and skeletal muscles [31]. Interestingly, in addition to the beneficial effect of Zygosids on blood glucose and insulin levels, an improvement in lipid profile was also observed. This feature supports the hypothesis on the mode of action of Zygosids.
- The excessive accumulation of the labile iron has been linked to pathologic processes in T2DM, resulting in T2DM complications in various organs, such as cardiomyopathy, peripheral neuropathy, nephropathy, and non-alcoholic fatty liver disease [12,32-34]. These phenomena were accompanied, or even initiated [35] by up-regulation in concentration of the proinflammatory cytokines, including those, tested in this study [5]. Therefore, sequestration of labile iron or even decrease in total amount of iron has beneficial effect, suppressing inflammation, IR, and mitigating T2DM complications [35]. It should be noted, that treatment with Zygosids showed an ability to reduce T2DM-induced fat accumulation within the liver.
- Furthermore, zinc supplementation was demonstrated to have certain antidiabetic and anti-inflammatory effects, and even partly alleviate IR [21,22]. Beneficial effects of zinc supplementation, partly mitigating several aspects of diabetes, e.g., certain reduction of BGL, improvement of lipoprotein profile, a statistically significant trend toward the decrease of glycosylated hemoglobin [35-37], and improvement of renal function, have been repeatedly reported [35-37]. In contrast, lower serum zinc level in T2DM patients was related to higher prevalence of diabetic microvascular complications, and was represented as an independent risk factor for diabetic nephropathy [38]. Patients with lower zinc level were more likely to have poorer glucose control, and worse β-cell function [38]. Yet, despite numerous attempts, the beneficial effect of zinc supplement to T2DM patients was auxiliary only, emphasizing the influence of additional factors.
- The association between the inflammatory process and accumulation of labile redox-active iron is much wider, reaching beyond T2DM. It is highly important to emphasize, that the triggering role of redox-active iron accumulation in inflammatory processes was observed in several pathologies. Analogous phenomena have been reported in a variety of other inflammatory conditions, i.e., inflammatory bowel disease [39], nasal polyposis [40], asthma [25], and even Parkinson’s disease [41]. Generally, one of the first responses, initiated by inflammatory conditions, independently of its source, is enhanced secretion of hepcidin, iron-regulating hormone, triggered by up-regulation of IL-6, IL-1α, and IL-1β, the proinflammatory cytokines. While the main purpose of this reaction is restriction of iron availability to bacterial pathogens, collaterally it results in accumulation of iron within different cells [42]. Since a fungicidal and anti-microbial activity of macrophages is based on intensive ROS production [42], while a certain amount of iron is required for dendritic cells maturation and lymphocytes proliferation [42], the process is critical for the immune system, but can be highly deleterious for other organs and tissues.
- The role, played by zinc, in broadly defined inflammatory processes, is also highly important. Overall, zinc deficiency results in immune dysfunction and promotes systemic inflammation [43]. In continuation to the above, zinc, inducing production of methallothioneins, is able to reduce the availability of redox-active metals, participating in ROS production [22]. On the other hand, zinc supplementation resulted in mitigation of the chronic low-grade inflammatory process through reduction in plasma C-reactive protein, TNF-α, and IL-6 concentrations [22]. Zinc ion by itself, inhibits the production of ROS [22,44]. Thus, since Zygosid-50 demonstrates an ability to act in a combined manner through both pathways, sequestrating the redox-active iron ion and providing zinc to the site of inflammation, its high antidiabetic efficacy on the animal model can be hypothesized. Therefore, the anti-T2DM effect of Zygosids is expressed in several aspects, i.e., suppression of the systemic low-grade chronic inflammation, normalization of BGL, significant reduction in serum insulin and improvement of the lipid profile. Interestingly, since Ga-DFO complex acts solely through the mechanism of iron chelation and its displacement of the redox-active ions by the relatively inert gallium ion at specific sites [45], without replenishing zinc deficiency, it can explain its lower efficacy when compared to Zn-DFO complex, Zygosid-50.
- We consider that the important finding of this study is a demonstration of the ability of the Zygosids not just to suppress the symptoms of T2DM but also to mitigate IR, the main pathologic causative feature of the disease. Based on all the above mentioned, an impressive therapeutic effect on human patients can be expected.
DISCUSSION
-
CONFLICTS OF INTEREST
Vladimir Vinokur and Dror Chevion are employees of Concenter Biopharma. They were not involved in the review process of this article. Otherwise, there was no conflict of interest.
-
AUTHOR CONTRIBUTIONS
Conception or design: V.V., E.B., M.C.
Acquisition, analysis, or interpretation of data: V.V., M.C.
Drafting the work or revising: V.V.
Final approval of the manuscript: D.C.
-
FUNDING
None
NOTES
-
Acknowledgements
- Part of the data was shown as a part of the poster or the lecture at the scientific meetings (https://www.metabolismjournal.com/article/S0026-0495(19)30310-5/fulltext).


- 1. O’Connell JM, Manson SM. Understanding the economic costs of diabetes and prediabetes and what we may learn about reducing the health and economic burden of these conditions. Diabetes Care 2019;42:1609-11.ArticlePubMedPMCPDF
- 2. GlobalData. Type 2 diabetes market to more than double, to $64 billion by 2026. Available from: https://www.globaldata.com/media/press-release/type-2-diabetes-market-double-64-billion-2026 (cited 2023 May 15).
- 3. Fernandez-Real JM, McClain D, Manco M. Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of type 2 diabetes. Diabetes Care 2015;38:2169-76.ArticlePubMedPDF
- 4. Chirumbolo S, Rossi AP, Rizzatti V, Zoico E, Franceschetti G, Girelli D, et al. Iron primes 3T3-L1 adipocytes to a TLR4-mediated inflammatory response. Nutrition 2015;31:1266-74.ArticlePubMed
- 5. Andrews M, Soto N, Arredondo-Olguin M. Association between ferritin and hepcidin levels and inflammatory status in patients with type 2 diabetes mellitus and obesity. Nutrition 2015;31:51-7.ArticlePubMed
- 6. MacDonald MJ, Cook JD, Epstein ML, Flowers CH. Large amount of (apo)ferritin in the pancreatic insulin cell and its stimulation by glucose. FASEB J 1994;8:777-81.ArticlePubMedPDF
- 7. Aigner E, Felder TK, Oberkofler H, Hahne P, Auer S, Soyal S, et al. Glucose acts as a regulator of serum iron by increasing serum hepcidin concentrations. J Nutr Biochem 2013;24:112-7.ArticlePubMed
- 8. Niederau C, Berger M, Stremmel W, Starke A, Strohmeyer G, Ebert R, et al. Hyperinsulinaemia in non-cirrhotic haemochromatosis: impaired hepatic insulin degradation? Diabetologia 1984;26:441-4.ArticlePubMedPDF
- 9. Davis RJ, Corvera S, Czech MP. Insulin stimulates cellular iron uptake and causes the redistribution of intracellular transferrin receptors to the plasma membrane. J Biol Chem 1986;261:8708-11.ArticlePubMed
- 10. Ferrannini E. Insulin resistance, iron, and the liver. Lancet 2000;355:2181-2.ArticlePubMed
- 11. Dongiovanni P, Ruscica M, Rametta R, Recalcati S, Steffani L, Gatti S, et al. Dietary iron overload induces visceral adipose tissue insulin resistance. Am J Pathol 2013;182:2254-63.ArticlePubMed
- 12. Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes 2002;51:2348-54.ArticlePubMedPDF
- 13. Cutler P. Deferoxamine therapy in high-ferritin diabetes. Diabetes 1989;38:1207-10.ArticlePubMed
- 14. Kaye TB, Guay AT, Simonson DC. Non-insulin-dependent diabetes mellitus and elevated serum ferritin level. J Diabetes Complications 1993;7:246-9.ArticlePubMed
- 15. De Sanctis V, Soliman A, Yassin M. Iron overload and glucose metabolism in subjects with β-thalassaemia major: an overview. Curr Diabetes Rev 2013;9:332-41.PubMed
- 16. Chevion M. Protection against free radical-induced and transition metal-mediated damage: the use of “pull” and “push” mechanisms. Free Radic Res Commun 1991;12-13 Pt 2:691-6.ArticlePubMed
- 17. Keberle H. The biochemistry of desferrioxamine and its relation to iron metabolism. Ann N Y Acad Sci 1964;119:758-68.ArticlePubMed
- 18. Hjorth CF, Norrman M, Wahlund PO, Benie AJ, Petersen BO, Jessen CM, et al. Structure, aggregation, and activity of a covalent insulin dimer formed during storage of neutral formulation of human insulin. J Pharm Sci 2016;105:1376-86.ArticlePubMed
- 19. Egefjord L, Petersen AB, Rungby J. Zinc, alpha cells and glucagon secretion. Curr Diabetes Rev 2010;6:52-7.ArticlePubMed
- 20. Norouzi S, Adulcikas J, Sohal SS, Myers S. Zinc transporters and insulin resistance: therapeutic implications for type 2 diabetes and metabolic disease. J Biomed Sci 2017;24:87.ArticlePubMedPMCPDF
- 21. Wu Y, Lu H, Yang H, Li C, Sang Q, Liu X, et al. Zinc stimulates glucose consumption by modulating the insulin signaling pathway in L6 myotubes: essential roles of Akt-GLUT4, GSK3β and mTOR-S6K1. J Nutr Biochem 2016;34:126-35.ArticlePubMed
- 22. Olechnowicz J, Tinkov A, Skalny A, Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci 2018;68:19-31.ArticlePubMedPMCPDF
- 23. Jansen J, Rosenkranz E, Overbeck S, Warmuth S, Mocchegiani E, Giacconi R, et al. Disturbed zinc homeostasis in diabetic patients by in vitro and in vivo analysis of insulinomimetic activity of zinc. J Nutr Biochem 2012;23:1458-66.ArticlePubMed
- 24. Kaiser N, Cerasi E, Leibowitz G. Diet-induced diabetes in the sand rat (Psammomys obesus). Methods Mol Biol 2012;933:89-102.ArticlePubMed
- 25. Bibi H, Vinokur V, Waisman D, Elenberg Y, Landesberg A, Faingersh A, et al. Zn/Ga-DFO iron-chelating complex attenuates the inflammatory process in a mouse model of asthma. Redox Biol 2014;2:814-9.ArticlePubMedPMC
- 26. Anis Y, Leshem O, Reuveni H, Wexler I, Ben Sasson R, Yahalom B, et al. Antidiabetic effect of novel modulating peptides of G-protein-coupled kinase in experimental models of diabetes. Diabetologia 2004;47:1232-44.ArticlePubMedPDF
- 27. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467-74.ArticlePubMed
- 28. Goebeler M, Gutwald J, Roth J, Sorg C. The severity of irritant contact dermatitis in various strains of mice correlates with endothelial expression of migration inhibitory factor (MIF). Arch Dermatol Res 1991;283:246-50.ArticlePubMedPDF
- 29. Ryan BJ, Van Pelt DW, Guth LM, Ludzki AC, Gioscia-Ryan RA, Ahn C, et al. Plasma ferritin concentration is positively associated with in vivo fatty acid mobilization and insulin resistance in obese women. Exp Physiol 2018;103:1443-7.ArticlePubMedPMCPDF
- 30. Liu P, Gan W, Inuzuka H, Lazorchak AS, Gao D, Arojo O, et al. Sin1 phosphorylation impairs mTORC2 complex integrity and inhibits downstream Akt signalling to suppress tumorigenesis. Nat Cell Biol 2013;15:1340-50.ArticlePubMedPMCPDF
- 31. Cui R, Choi SE, Kim TH, Lee HJ, Lee SJ, Kang Y, et al. Iron overload by transferrin receptor protein 1 regulation plays an important role in palmitate-induced insulin resistance in human skeletal muscle cells. FASEB J 2019;33:1771-86.ArticlePubMedPDF
- 32. Koenig G, Seneff S. Gamma-glutamyltransferase: a predictive biomarker of cellular antioxidant inadequacy and disease risk. Dis Markers 2015;2015:818570.ArticlePubMedPMCPDF
- 33. Sun L, Qi Q, Zong G, Ye X, Li H, Liu X, et al. Elevated plasma retinol-binding protein 4 is associated with increased risk of type 2 diabetes in middle-aged and elderly Chinese adults. J Nutr 2014;144:722-8.ArticlePubMed
- 34. Juanola-Falgarona M, Candido-Fernandez J, Salas-Salvado J, Martinez-Gonzalez MA, Estruch R, Fiol M, et al. Association between serum ferritin and osteocalcin as a potential mechanism explaining the iron-induced insulin resistance. PLoS One 2013;8:e76433.ArticlePubMedPMC
- 35. Wieser V, Moschen AR, Tilg H. Inflammation, cytokines and insulin resistance: a clinical perspective. Arch Immunol Ther Exp (Warsz) 2013;61:119-25.ArticlePubMedPDF
- 36. Ruz M, Carrasco F, Rojas P, Codoceo J, Inostroza J, Basfi-fer K, et al. Zinc as a potential coadjuvant in therapy for type 2 diabetes. Food Nutr Bull 2013;34:215-21.ArticlePubMedPDF
- 37. Prasad AS. Zinc is an antioxidant and anti-inflammatory agent: its role in human health. Front Nutr 2014;1:14.ArticlePubMedPMC
- 38. Luo YY, Zhao J, Han XY, Zhou XH, Wu J, Ji LN. Relationship between serum zinc level and microvascular complications in patients with type 2 diabetes. Chin Med J (Engl) 2015;128:3276-82.ArticlePubMedPMC
- 39. Chieppa M, Galleggiante V, Serino G, Massaro M, Santino A. Iron chelators dictate immune cells inflammatory ability: potential adjuvant therapy for IBD. Curr Pharm Des 2017;23:2289-98.ArticlePubMed
- 40. Vinokur V, Berenshtein E, Chevion MM, Eliashar R. Iron homeostasis and methionine-centred redox cycle in nasal polyposis. Free Radic Res 2011;45:366-73.ArticlePubMed
- 41. Phillipson OT. Management of the aging risk factor for Parkinson’s disease. Neurobiol Aging 2014;35:847-57.ArticlePubMed
- 42. Martins AC, Almeida JI, Lima IS, Kapitao AS, Gozzelino R. Iron metabolism and the inflammatory response. IUBMB Life 2017;69:442-50.ArticlePubMedPDF
- 43. Wong CP, Rinaldi NA, Ho E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol Nutr Food Res 2015;59:991-9.ArticlePubMedPMCPDF
- 44. Li HT, Jiao M, Chen J, Liang Y. Roles of zinc and copper in modulating the oxidative refolding of bovine copper, zinc superoxide dismutase. Acta Biochim Biophys Sin (Shanghai) 2010;42:183-94.ArticlePubMedPDF
- 45. Banin E, Berenshtein E, Kitrossky N, Pe’er J, Chevion M. Gallium-desferrioxamine protects the cat retina against injury after ischemia and reperfusion. Free Radic Biol Med 2000;28:315-23.ArticlePubMed
REFERENCES
Figure & Data
References
Citations


 KDA
KDA