- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 46(4); 2022 > Article
-
Original ArticleComplications Advanced Liver Fibrosis Is Associated with Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease
-
Da Hea Seo1
 , Young Ju Suh2, Yongin Cho1, Seong Hee Ahn1, Seongha Seo1, Seongbin Hong1, Yong-ho Lee3, Young Ju Choi4, Eunjig Lee3, So Hun Kim1
, Young Ju Suh2, Yongin Cho1, Seong Hee Ahn1, Seongha Seo1, Seongbin Hong1, Yong-ho Lee3, Young Ju Choi4, Eunjig Lee3, So Hun Kim1
-
Diabetes & Metabolism Journal 2022;46(4):630-639.
DOI: https://doi.org/10.4093/dmj.2021.0130
Published online: January 26, 2022
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Inha University College of Medicine, Incheon, Korea
2Department of Biomedical Sciences, Inha University College of Medicine, Incheon, Korea
3Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
4Huh’s Diabetes Center, Seoul, Korea
-
Corresponding author: So Hun Kim
 Division of Endocrinology and Metabolism, Department of Internal Medicine, Inha University College of Medicine, 27 Inhang-ro, Jung-gu, Incheon 22332, Korea E-mail: shoney@inha.ac.kr
Division of Endocrinology and Metabolism, Department of Internal Medicine, Inha University College of Medicine, 27 Inhang-ro, Jung-gu, Incheon 22332, Korea E-mail: shoney@inha.ac.kr
Copyright © 2022 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Nonalcoholic fatty liver disease (NAFLD) is associated with chronic kidney disease (CKD). However, the causal relationship between NAFLD and CKD is uncertain, particularly in patients with type 2 diabetes mellitus (T2DM). We aimed to investigate the association between the presence and severity of NAFLD and incident CKD in patients with T2DM.
-
Methods
- In this longitudinal cohort study of patients with T2DM, 3,188 patients with preserved renal function were followed up for the occurrence of incident CKD. NAFLD was defined as the presence of hepatic steatosis on ultrasonography, without any other causes of chronic liver disease. Advanced liver fibrosis of NAFLD was defined as a fibrosis-4 index ≥2.67. CKD was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2.
-
Results
- At baseline, 1,729 (54.2%) patients had NAFLD, of whom 94 (5.4%) had advanced liver fibrosis. During the follow-up of 8.3±3.6 years, 472 (14.8%) patients developed incident CKD: 220 (15.1%) in the non-NAFLD group, 231 (14.1%) in the NAFLD without advanced fibrosis group and 28 (31.1%) in the NAFLD with advanced fibrosis group. There was no increased risk of incident CKD in the NAFLD group compared to the non-NAFLD group (P=0.435). However, among patients with NAFLD, advanced liver fibrosis was associated with an increased risk of CKD (adjusted hazard ratio, 1.75; 95% confidence interval, 1.15 to 2.66; P=0.009).
-
Conclusion
- Advanced liver fibrosis in patients with NAFLD is independently associated with an increased risk of incident CKD in patients with T2DM.
- Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide with a prevalence of 10% to 30% in the general population [1]. The incidence of NAFLD is expected to increase rapidly with an increase in the prevalence of obesity, especially in Asia [2]. NAFLD encompasses a broad spectrum of liver diseases ranging from simple steatosis to nonalcoholic steatohepatitis (NASH) and liver cirrhosis [3]. NAFLD is highly prevalent in patients with type 2 diabetes mellitus (T2DM), with a prevalence of 50% to 75% [4,5]. Risk factors associated with the progression to NASH or advanced liver fibrosis have been widely investigated, and T2DM is a significant predictor of hepatic fibrosis [6,7]. The presence of advanced liver fibrosis is clinically important because it is associated with an increased risk of liver transplantation, liver-related mortality, cardiovascular outcomes, and all-cause mortality in patients with NAFLD [8,9].
- There is growing evidence suggesting a possible link between NAFLD and chronic kidney disease (CKD). NAFLD and CKD share some common features, including visceral obesity, metabolic syndrome, and increased risk of cardiovascular disease [10,11]. Several cross-sectional and longitudinal studies mainly involving patients without diabetes have shown that the presence and severity of NAFLD is associated with an increased risk of CKD [12–14]. However, limited studies have been conducted on patients with diabetes [15–19]. To date, only two longitudinal studies have examined the association between NAFLD and incident CKD in patients with diabetes, which demonstrated that NAFLD is associated with an increased incidence of CKD [15,16]. However, both studies examined cohorts of Italian patients, and the association between the severity of NAFLD and the risk of incident CKD in patients with diabetes was not studied. Therefore, this longitudinal study aimed to evaluate whether the presence of NAFLD or advanced liver fibrosis in NAFLD is independently associated with the development of incident CKD in a large cohort of Asian patients with T2DM and preserved renal function.
INTRODUCTION
- Study population
- A total of 13,296 patients from the Seoul Metabolic Syndrome cohort were enrolled between January 2000 and December 2016 at the Huh Diabetes Center in Seoul, Republic of Korea. Patients aged <19 years and with type 1 diabetes mellitus (T1DM, n=133), those with missing data on baseline abdominal ultrasonography (n=3,602) and laboratory results including renal function tests and rate constant for plasma glucose disappearance (KITT, n=4,940), those with daily alcohol consumption >30 g for men and >20 g for women or with other causes of liver disease (n=264), those with an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 (n= 918), and those with <3 annual follow-up visits with renal function assessment (n=251) were excluded from the study (Supplementary Fig. 1). Finally, 3,188 patients who had preserved renal function (eGFR ≥60 mL/min/1.73 m2) at baseline and complete information on all covariates including at least three consecutive annual renal function assessments and who underwent both short insulin tolerance test (SITT) and abdominal ultrasonography were included in the analyses. The interval between each visit varied among patients, and the last follow-up was conducted in December 2019. All participants provided written informed consent, and the study was approved by the Institutional Review Board of Inha University Hospital (IRB no. 2020-04-037).
- Measurements of clinical and laboratory indices
- Anthropometric indices, including weight, height, and waist circumference (WC), were measured in all patients by a well-trained nurse who was blinded to the patients’ clinical and laboratory data. WC was measured at the midpoint of the lower ribs and the iliac crest at the end of the expiratory phase. All patients underwent blood tests to measure the metabolic parameters including fasting plasma glucose, glycosylated hemoglobin (HbA1c), C-peptide, insulin, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), and high-sensitivity C-reactive protein (hs-CRP) levels; renal and liver function tests, including plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels; and complete blood count assessment including platelet count. Data on patients’ social and medical histories were collected using a self-questionnaire.
- Definition of incident chronic kidney disease
- The eGFR values were calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation [20]: eGFR=141×min (Scr/κ, 1)α×max (Scr/κ, 1)−1.209×0.993Age×1.018 (if female), where Scr is the serum creatinine level in mg/dL, κ is 0.7 for women and 0.9 for men, α is −0.329 for women and −0.411 for men, min is the minimum value of Scr/κ or 1, and max is the maximum value of Scr/κ or 1. Incident CKD was defined as an eGFR of <60 mL/min/1.73 m2 for two consecutive times during follow-up visits.
- Short insulin tolerance test
- SITT was performed to assess the insulin sensitivity. The KITT (%/min) was used as a marker of insulin sensitivity [21]. As previously described [22], the test was performed at 8:00 AM after an 8-hour fasting. Venous blood samples were collected at 0, 3, 6, 9, 12, and 15 minutes after an intravenous bolus injection of regular insulin (Humulin, Eli Lilly, Indianapolis, IN, USA) at a dose of 0.1 U/kg. Plasma glucose levels were measured immediately after sampling using a Beckman Glucose Analyzer II (Beckman Instruments, Fullerton, CA, USA). After the test, 100 mL of 20% dextrose solution was immediately administered intravenously to prevent potential hypoglycemia. The KITT value was calculated from the slope of the fall in the log-transformed plasma glucose level from 3 to 15 minutes, which was used to calculate the time taken for the basal level of blood glucose to decrease by half (t1/2). The formula used was KITT=0.693/t1/2×100 (%/min). Higher KITT values indicate higher insulin sensitivity.
- Assessment of NAFLD and definition of advanced liver fibrosis
- Abdominal ultrasonography was performed by a well-trained radiologist using a high-resolution ultrasonography device with a 3.5-MHz transducer (iU22, Philips Healthcare, Andover, MA, USA) after an 8-hour fast. NAFLD was defined as the presence of hepatic steatosis on ultrasonography based on the following sonographic features: hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring [23]. The NAFLD fibrosis score (NFS) and fibrosis-4 (FIB-4) index were selected as surrogate indices for defining severe NAFLD with advanced liver fibrosis. Calculations of NFS and FIB-4 were conducted only in patients with NAFLD. The following equations were used to calculate these indices: NFS=−1.675+ 0.037×age (years)+0.094× body mass index (BMI, kg/m2)+ 1.13×impaired fasting glucose or diabetes (yes=1, no=0)+ 0.99×AST/ALT ratio−0.013×platelet count (×109/L)−0.66× serum albumin [g/dL]; and FIB-4=(age [years]×AST [U/L])/(platelets [109/L]×[ALT (U/L)]1/2) [24]. Significant fibrosis was defined as an NFS >0.675 or FIB-4 ≥2.67 [24]. To test the robustness of our findings, we also used the hepatic steatosis index (HSI) to define NAFLD and those with HSI >36 were considered to have NAFLD: HSI=8×(ALT/AST ratio)+BMI (+2, if female; +2, if diabetes mellitus) [25].
- Statistical analysis
- Continuous variables are reported as mean±standard deviation or medians with interquartile ranges, while categorical variables are reported as numbers and percentages. The patients were grouped according to the presence and severity of NAFLD as non-NAFLD (patients without NAFLD), NAFLD without advanced fibrosis, and NAFLD with advanced liver fibrosis. To evaluate the differences in demographic characteristics between the non-NAFLD, NAFLD without advanced fibrosis, and NAFLD with advanced liver fibrosis groups, analysis of variance with Bonferroni correction was used for continuous variables and the chi-square test was used for categorical variables. The baseline characteristics according to the presence of NAFLD or incident CKD were compared using Student’s t-test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables as appropriate. Cumulative event rates for incident CKD were estimated using Kaplan–Meier survival curves and the log-rank test. The Cox proportional hazards model was used to determine the independent association between NAFLD or advanced liver fibrosis and incident CKD after adjusting for confounding variables including age, sex, BMI, duration of diabetes, hypertension (HTN), systolic blood pressure (SBP), baseline HbA1c level, total cholesterol level, eGFR, use of sulfonylurea, insulin, statin, and angiotensin converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB), hs-CRP levels, and KITT values. The adjusted hazard ratios (HRs) were estimated using 95% confidence intervals (CIs). Statistical significance was set at P<0.05. All statistical analyses were performed using SPSS statistical software version 19.0 (IBM Co., Armonk, NY, USA).
METHODS
- Baseline characteristics of the study population according to the presence of NAFLD
- As shown in Table 1, a total of 3,188 people were included in the study; the mean age was 56.7±10.1 years, 685 participants (46.9%) were male, and the mean duration of diabetes was 7.4±6.8 years. The prevalence of NAFLD was 54.2%. Compared with patients without NAFLD, those with NAFLD were younger, more likely to be men, and had a shorter duration of diabetes. In addition, patients with NAFLD had a poorer metabolic profile with higher BMI, WC, SBP, hs-CRP levels, total cholesterol levels, TG levels, and LDL-C levels and lower KITT values and HDL-C levels than those without NAFLD (P<0.05) (Supplementary Table 1). Patients with NAFLD had higher AST and ALT levels than those without NAFLD. Baseline eGFR did not differ between patients with and without NAFLD.
- Baseline characteristics of the study population according to the severity of NAFLD
- Patients were stratified according to the severity of NAFLD using a combination of abdominal ultrasonography and FIB-4 index: 1,459 (45.8%) did not have NAFLD (non-NAFLD), 1,635 (51.3%) had NAFLD without advanced fibrosis, and 94 (2.9%) had NAFLD with advanced liver fibrosis (Table 1). Compared with patients without NAFLD, those with NAFLD with advanced liver fibrosis were older; had higher BMI, WC, SBP, triglyceride, AST, and hs-CRP levels; and lower KITT values, and HDL-C levels. Moreover, patients with NAFLD with advanced liver fibrosis were more likely to have HTN and use sulfonylurea, ACE inhibitors, or ARBs than those without NAFLD. Among patients with NAFLD, those with advanced liver fibrosis were older and had higher BMI, WC, SBP, and AST levels than those without advanced liver fibrosis. The use of sulfonylurea, thiazolidinediones, ACE inhibitors, or ARBs was higher in patients with advanced liver fibrosis than in those without advanced liver fibrosis. When NAFLD was defined using HSI, similar findings were observed (Supplementary Table 2).
- Risk of incident CKD according to the severity of NAFLD
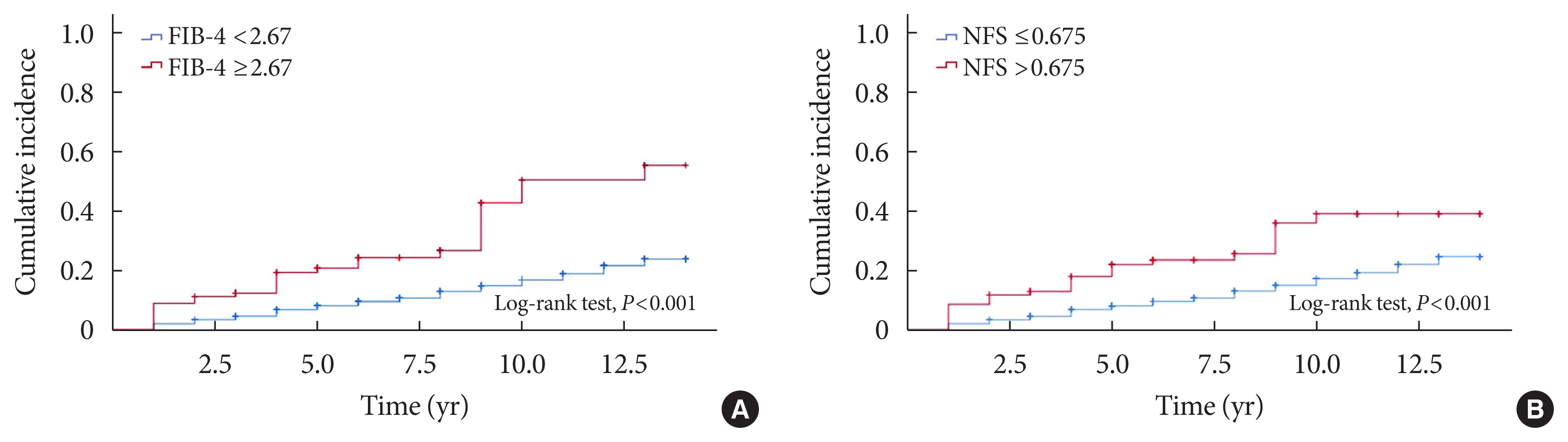
- During the mean follow-up duration of 8.3±3.6 years, 472 (14.8%) patients developed incident CKD. The cumulative incidence rates of CKD were 220 (15.1%) in the non-NAFLD group, 226 (12.8%) in the NAFLD without advanced fibrosis group, and 26 (27.7%) in the NAFLD with advanced fibrosis group. Table 2 shows the baseline characteristics of the patients according to the development of incident CKD. Compared with patients who did not develop CKD, those who developed CKD were older, more likely to be women, had a longer duration of diabetes, and had lower eGFR levels at baseline. In addition, patients with incident CKD had a poorer metabolic profile, with higher SBP, HbA1c, and hs-CRP levels, and lower KITT values. Patients who developed CKD had a higher prevalence of HTN and were more likely to be treated with insulin, sulfonylureas, or statins at baseline than those who did not develop CKD (Table 2). The cumulative incidence of CKD was not significantly different between patients with and without NAFLD. Among patients with NAFLD, the cumulative incidence of CKD was significantly higher in patients with advanced liver fibrosis than in those without advanced liver fibrosis (P<0.0001 by log-rank test) (Fig. 1A). Almost identical results were found when NFS was used to estimate advanced liver fibrosis among patients with NAFLD (Fig. 1B).
- To further examine the relationship between NAFLD and incident CKD, the Cox proportional hazards model was used. No significant association was observed between NAFLD and incident CKD (Table 3). However, in patients with NAFLD, the presence of advanced liver fibrosis was associated with a significantly increased risk of incident CKD after adjusting for age, sex, and BMI (Model 1: HR, 1.96; 95% CI, 1.32 to 2.92; P= 0.001). The association remained significant after further adjustment for clinical risk factors including duration of diabetes, HTN, SBP, baseline HbA1c level, total cholesterol level, eGFR, and medications (sulfonylurea, insulin, statin, ACE inhibitors, or ARBs). Finally, this association remained robust even after further adjustment for hs-CRP level and KITT value (Model 4: HR, 1.75; 95% CI, 1.15 to 2.66; P=0.009) (Table 4). When advanced liver fibrosis was assessed using the NFS, a similar association was observed between advanced liver fibrosis and incident CKD (Table 4). When NAFLD was defined using HSI, these findings were consistent (Supplementary Table 3).
RESULTS
- In this longitudinal study with a large cohort of Korean patients with T2DM and preserved renal function at baseline, we found that advanced liver fibrosis in NAFLD was associated with an increased risk of incident CKD during a mean follow-up of 8.3 years. However, there was no association between the presence of NAFLD and CKD incidence. The association between advanced liver fibrosis in NAFLD and incident CKD seems to be independent of numerous confounding factors including age, sex, BMI, duration of diabetes, SBP, baseline HbA1c, total cholesterol, and eGFR levels, use of sulfonylurea, insulin, statin, ACE inhibitors or ARB, hs-CRP level, and KITT value.
- There has been growing epidemiological evidence suggesting a link between NAFLD and CKD in the general population. However, the results have been inconclusive due to differences in the study populations, diagnostic modalities to detect NAFLD, and outcome definitions [12,13]. In recent meta-analyses, NAFLD was associated with a 1.3 to 2.1-fold increased risk of prevalent CKD and incident CKD, and the risk of incident CKD increased progressively with increased NAFLD severity [14,26]. However, in a large Asian cohort study using non-invasive fibrosis markers, the increased risk of incident CKD was restricted to patients with advanced liver fibrosis [27]. Only a few studies have examined the association between NAFLD and CKD in the diabetic population. In two cross-sectional studies by Targher et al. [17,18], the presence of NAFLD was associated with an increased risk of CKD in patients with T1DM and T2DM. Similarly, in a prospective study of 1,827 patients with T2DM, ultrasound-diagnosed NAFLD was associated with a 1.6-fold increased risk of incident CKD [15]. Overall, the direction of NAFLD-related effects on the risk of prevalent or incident CKD seems to be similar, but with greater magnitude than that observed in the population without diabetes. However, previous studies with a diabetic population did not assess the association between the severity of NAFLD and the risk of incident CKD. In the current study, we demonstrated that only the severe form of NAFLD was associated with a 1.7 to 1.9-fold increased risk of incident CKD in patients with T2DM. This relationship is similar to that observed in a comprehensive meta-analysis, which demonstrated a strong link between the severity of NAFLD and increased risk of fatal and nonfatal cardiovascular outcomes [8,19]. The association between advanced liver fibrosis and CKD is further supported by a clinical trial involving patients with biopsy-proven NASH, in which improvement of liver fibrosis stages with lifestyle modification was independently associated with improved renal function [28].
- The underlying mechanisms linking NAFLD and CKD are not fully understood. However, accumulating evidence suggests that NAFLD is involved in the pathogenesis of CKD, with systemic release of multiple mediators including increased reactive oxygen species, advanced glycation end products, hs-CRP, and pro-inflammatory, pro-fibrogenic, and anti-fibrinolytic molecules including fibroblast growth factor-21, tumor necrosis factor-α, and transforming growth factor-β (TGFβ), all of which can promote kidney injury [29,30]. It has also been suggested that insulin resistance, atherogenic dyslipidemia, renin-angiotensin-aldosterone system activity, and release of pro-inflammatory cytokines through activation of hepatic macrophages and hepatic inflammation are contributing mechanisms linking NAFLD and CKD [31]. Findings from our study and previous studies suggest that liver fibrosis and its progression in NAFLD play a key role in the development of CKD [10,32]. Although not completely understood, TGFβ is the most potent fibrogenic cytokine and a key driver of liver fibrosis [33] and CKD progression [34]. Thus, we can speculate that TGFβ may act as a link between liver fibrosis in NAFLD and CKD progression, even in patients with T2DM. Further studies are warranted to elucidate the underlying mechanisms.
- Advanced liver fibrosis is a strong predictor of all-cause mortality, liver-related mortality, and liver-related events in patients with NAFLD [9]. It is particularly important in patients with diabetes, as comorbid metabolic abnormalities are associated with a significantly higher risk of advanced liver fibrosis among patients with NAFLD [35]. In addition to the known risks of advanced liver fibrosis, there is also a higher risk of developing CKD in such patients, as reported in our study, indicating the need for surveillance and preventive measures for CKD in patients with advanced liver fibrosis and T2DM.
- This study has some strengths. This is a longitudinal study that involved serial measurements of kidney function to accurately identify incident CKD during 8.3±3.6-year follow-up. We only included patients with preserved renal function to assess the association between baseline severity of NAFLD and incident CKD. Moreover, our cohort consisted of a large number of participants from a homogeneous population with >8 years of follow-up. We also measured the degree of insulin resistance using a standard KITT method and included a broad spectrum of clinical variables as clinical risk factors in our analysis. To our knowledge, this is the first longitudinal study to report that the severe form of NAFLD is associated with an increased risk of incident CKD in Asian patients with T2DM and preserved renal function.
- However, this study has some limitations. First, we used abdominal ultrasonography to assess the presence of NAFLD instead of liver biopsy, which is the gold standard for the diagnosis of NAFLD. Moreover, we used fibrosis prediction models instead of histological confirmation to assess the severity of NAFLD. For this large cohort of patients with T2DM, it would be unethical to perform an invasive liver biopsy with histological confirmation in asymptomatic patients. Abdominal ultrasonography is more frequently used owing to its low cost, easy accessibility, and noninvasiveness [23]. In clinical and population settings, ultrasonography is likely the imaging technique of choice for screening of NAFLD, and fibrosis prediction models have been well validated in different patient populations and ethnicities [36]. Second, we did not measure any biomarkers of fibrosis in NAFLD to support the link between CKD and advanced liver fibrosis in patients with T2DM. Lastly, we did not have data on albuminuria, which is an important marker of diabetic nephropathy. However, it is still valuable to assess the risk factors that can lead to a reduction in renal function in patients with diabetes. Therefore, our findings may have an additive role in identifying individuals at risk for CKD.
- In conclusion, in a large cohort of Korean patients with T2DM, we demonstrated that advanced liver fibrosis is associated with an increased risk of incident CKD, independent of traditional risk factors. In addition to its role as a monitoring tool for hepatic disease progression, the fibrosis prediction models in patients with diabetes and NAFLD may also serve as a tool to identify those at risk of extrahepatic complications, in this case, CKD in patients with T2DM. Future prospective trials are warranted to better understand the natural course of CKD associated with longitudinal dynamic changes in NAFLD in patients with T2DM.
DISCUSSION
SUPPLEMENTARY MATERIALS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: D.H.S., S.H.K.
Acquisition, analysis, or interpretation of data: D.H.S., Y.J.S., S.H.K.
Drafting the work or revising: D.H.S., Y.C., S.H.A., S.S., S.H., Y.L., Y.J.C., E.L.
Final approval of the manuscript: S.H.K.
-
FUNDING
This study was supported by a research grant from Inha University. The study sponsor/funder was not involved in the design of the study, the collection, analysis, and interpretation of data, writing the report, and no restrictions were imposed regarding the publication of the report.
NOTES
-
Acknowledgements
- The authors thank Dr. Kap Bum Huh for his dedication and contribution to this research.
ACKNOWLEDGMENTS
| Characteristic | Non-NAFLD (n=1,459) | NAFLD without advanced fibrosis (n=1,639) | NAFLD with advanced liver fibrosis (n=90) | P value |
|---|---|---|---|---|
| Age, yr | 57.6±10.2 | 55.4±9.9a | 64.8±7.2a,b | 0.039c |
| Male sex | 685 (46.9) | 863 (52.7) | 49 (54.4) | 0.005c |
| Duration of diabetes, yr | 8.4±7.4 | 6.4±6.0a | 8.1±7.3 | <0.001c |
| BMI, kg/m2 | 23.0±2.8 | 25.6±2.9a | 26.3±2.7a,b | <0.001c |
| WC, cm | 79.5±7.9 | 86.9±7.6a | 89.9±7.0a,b | <0.001c |
| SBP, mm Hg | 132.8±18.3 | 135.8±16.9a | 142.3±18.4a,b | <0.001c |
| DBP, mm Hg | 90.9±275.7 | 87.0±11.0 | 86.3±12.3 | 0.565 |
| FPG, mg/dL | 158.6±61.5 | 161.0±55.1 | 161.1±56.5 | 0.263 |
| HbA1c, % | 8.3±2.1 | 8.4±1.8 | 8.1±1.7 | 0.606 |
| KITT, %/min | 2.3±1.0 | 1.9±0.8a | 1.7±0.8a | <0.001c |
| Total cholesterol, mg/dL | 189.4±37.8 | 201.9±41.8a | 194.0±37.6 | <0.001c |
| Triglyceride, mg/dL | 114.9±68.8 | 175.1±132.6a | 158.1±90.8a | <0.001c |
| HDL-C, mg/dL | 54.3±14.8 | 48.7±12.0a | 48.8±13.2a | <0.001c |
| LDL-C, mg/dL | 112.6±32.6 | 119.2±37.3a | 114.6±34.4 | <0.001c |
| AST, IU/L | 25.8±13.0 | 28.5±12.2a | 56.1±46.9a,b | <0.001c |
| ALT, IU/L | 24.7±17.5 | 34.0±25.6a | 52.4±69.5a,b | <0.001c |
| eGFR, mL/min/1.73 m2 | 90.9±15.7 | 91.8±15.4 | 86.3±14.3 | 0.924 |
| Hs-CRP, mg/dL | 0.6 (0.3–1.1) | 1.0 (0.5–2.0)a | 1.0 (0.6–2.2)a | <0.001c |
| Uric acid, mg/dL | 4.2 ±1.3 | 4.6±1.4a | 4.6±1.3a | <0.001c |
| Hypertension | 358 (24.5) | 493 (30.1)a | 34 (37.8)a | <0.001c |
| Insulin | 204 (14.0) | 131 (8.0)a | 13 (14.4) | <0.001c |
| Metformin | 541 (37.1) | 815 (49.7)a | 26 (28.9) | <0.001c |
| Sulfonylurea | 739 (50.7) | 875 (53.4) | 51 (56.7)a,b | 0.218 |
| Thiazolidinedione | 160 (11.0) | 125 (7.6)a | 10 (11.1)b | 0.005c |
| ARB/ACE inhibitors | 60 (4.1) | 106 (6.5)a,b | 10 (11.1)a,b | 0.001c |
| Statin | 177 (12.1) | 320 (19.5)a | 8 (8.9)b | <0.001c |
| Incident CKD | 220 (15.1) | 231 (14.1) | 28 (31.1)a,b | <0.001c |
Values are presented as mean±standard deviation, number (%), or median (interquartile range).
NAFLD, nonalcoholic fatty liver disease; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; KITT, rate constant for plasma glucose disappearance; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate; Hs-CRP, high-sensitivity C-reactive protein; ARB, angiotensin II receptor blockers; ACE, angiotensin converting enzyme; CKD, chronic kidney disease.
a Indicates P<0.05 compared with NAFLD (−),
b Indicates P<0.05 compared with NAFLD without advanced liver fibrosis,
c Statistically significant.
| Characteristic | No CKD (n=2,716) | Incident CKD (n=472) | P value |
|---|---|---|---|
| Age, yr | 55.5±10.1 | 63.4±7.6 | <0.001a |
| Male sex | 1,426 (52.5) | 170 (36.0) | <0.001a |
| Duration of diabetes, yr | 6.9±6.5 | 10.2±7.5 | <0.001a |
| BMI, kg/m2 | 24.4±3.2 | 24.6±3.0 | 0.175 |
| WC, cm | 83.6±8.8 | 84.0±7.6 | 0.300 |
| SBP, mm Hg | 133.2±17.2 | 142.8±18.2 | <0.001a |
| DBP, mm Hg | 85.4±11.0 | 108.4±48.3 | 0.303 |
| FPG, mg/dL | 159.5±57.9 | 162.0±59.2 | 0.395 |
| HbA1c, % | 8.3±1.9 | 8.6±1.9 | 0.003a |
| KITT, %/min | 2.1±1.0 | 1.9±0.9 | <0.001a |
| Total cholesterol, mg/dL | 195.7±40.1 | 197.1±41.7 | 0.473 |
| Triglyceride, mg/dL | 145.5±110.0 | 156.1±116.3 | 0.055 |
| HDL-C, mg/dL | 51.4±13.6 | 50.5±13.8 | 0.187 |
| LDL-C, mg/dL | 116.0±34.9 | 116.0±37.4 | 0.993 |
| AST, IU/L | 28.0±15.8 | 28.3±13.4 | 0.683 |
| ALT, IU/L | 30.5±25.9 | 28.8±22.2 | 0.130 |
| eGFR, mL/min/1.73 m2 | 92.9±15.1 | 81.3±14.2 | <0.001a |
| Hs-CRP, mg/dL | 0.8 (0.4–1.6) | 0.9 (0.5–2.2) | <0.001a |
| Uric acid, mg/dL | 4.4±1.3 | 4.7±1.5 | <0.001a |
| Hypertension | 690 (25.4) | 195 (41.3) | <0.001a |
| Insulin | 257 (9.5) | 91 (19.3) | <0.001a |
| Metformin | 1,158 (42.6) | 224 (47.5) | 0.057 |
| Sulfonylurea | 1,365 (50.3) | 300 (63.6) | <0.001a |
| Thiazolidinedione | 251 (9.2) | 44 (9.3) | 1.000 |
| ARB/ACE inhibitors | 149 (5.5) | 27 (5.7) | 0.924 |
| Statin | 400 (14.7) | 105 (22.2) | <0.001a |
| NAFLD (+) | 1,476 (54.4) | 252 (53.4) | 0.732 |
Values are presented as mean±standard deviation, number (%), or median (interquartile range).
CKD, chronic kidney disease; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; KITT, rate constant for plasma glucose disappearance; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate; Hs-CRP, high-sensitivity C-reactive protein; ARB, angiotensin II receptor blocker; ACE, angiotensin converting enzyme; NAFLD, nonalcoholic fatty liver disease.
a Statistically significant.
| HR | 95% CI | P value | |
|---|---|---|---|
| Crude hazard ratio | 0.98 | 0.82–1.18 | 0.849 |
| Model 1 | 1.09 | 0.91–1.30 | 0.368 |
| Model 2 | 1.13 | 0.94–1.36 | 0.194 |
| Model 3 | 1.13 | 0.93–1.36 | 0.212 |
| Model 4 | 1.08 | 0.89–1.32 | 0.435 |
Model 1: adjustment for age (age was applied as a categorical variable with median age of 58 years), sex, and body mass index; Model 2: Model 1+adjustment for duration of diabetes, systolic blood pressure, hypertension, glycosylated hemoglobin level, total cholesterol level, and estimated glomerular filtration rate; Model 3: Model 2+adjustments for use of sulfonylurea, insulin, statin, and angiotensin converting enzyme inhibitor or angiotensin II receptor blockers; and Model 4: Model 3+adjustments for log high-sensitivity C-reactive protein level and rate constant for plasma glucose disappearance (KITT) value.
NAFLD, nonalcoholic fatty liver disease; CKD, chronic kidney disease; HR, hazard ratio; CI, confidence interval.
| By FIB-4 | By NFS | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Crude hazard ratio | 2.86 | 1.93–4.23 | <0.001a | 2.32 | 1.55–3.48 | <0.001a |
|
|
||||||
| Model 1 | 1.96 | 1.32–2.92 | 0.001a | 1.52 | 1.01–2.29 | 0.046a |
|
|
||||||
| Model 2 | 1.88 | 1.26–2.81 | 0.002a | 1.50 | 1.00–2.27 | 0.056 |
|
|
||||||
| Model 3 | 1.84 | 1.23–2.75 | 0.003a | 1.49 | 0.98–2.27 | 0.063 |
|
|
||||||
| Model 4 | 1.75 | 1.15–2.66 | 0.009a | 1.58 | 1.03–2.41 | 0.035a |
Model 1: adjustment for age (age was applied as a categorical variable with median age of 58 years), sex, and body mass index; Model 2: Model 1+adjustment for duration of diabetes, systolic blood pressure, hypertension, glycosylated hemoglobin level, total cholesterol level, and estimated glomerular filtration rate; Model 3: Model 2+adjustments for use of sulfonylurea, insulin, statin, and angiotensin converting enzyme inhibitor or angiotensin II receptor blockers; and Model 4: Model 3+adjustments for log high-sensitivity C-reactive protein level and rate constant for plasma glucose disappearance (KITT) value.
FIB-4, fibrosis-4; NFS, NAFLD fibrosis score; CKD, chronic kidney disease; NAFLD, nonalcoholic fatty liver disease; HR, hazard ratio; CI, confidence interval.
a Statistically significant.
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84.ArticlePubMed
- 2. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019;4:389-98.ArticlePubMed
- 3. Liou I, Kowdley KV. Natural history of nonalcoholic steatohepatitis. J Clin Gastroenterol 2006;40(Suppl 1):S11-6.PubMed
- 4. Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care 2017;40:419-30.ArticlePubMedPDF
- 5. Lee YH, Cho Y, Lee BW, Park CY, Lee DH, Cha BS, et al. Nonalcoholic fatty liver disease in diabetes. Part I: epidemiology and diagnosis. Diabetes Metab J 2019;43:31-45.ArticlePubMedPMCPDF
- 6. Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 2016;65:1359-68.ArticlePubMed
- 7. Koehler EM, Plompen EP, Schouten JN, Hansen BE, Darwish Murad S, Taimr P, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology 2016;63:138-47.ArticlePubMedPDF
- 8. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol 2016;65:589-600.ArticlePubMed
- 9. Taylor RS, Taylor RJ, Bayliss S, Hagstrom H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology 2020;158:1611-25.ArticlePubMed
- 10. Yasui K, Sumida Y, Mori Y, Mitsuyoshi H, Minami M, Itoh Y, et al. Nonalcoholic steatohepatitis and increased risk of chronic kidney disease. Metabolism 2011;60:735-9.ArticlePubMed
- 11. Kiapidou S, Liava C, Kalogirou M, Akriviadis E, Sinakos E. Chronic kidney disease in patients with non-alcoholic fatty liver disease: what the hepatologist should know? Ann Hepatol 2020;19:134-44.ArticlePubMed
- 12. Sirota JC, McFann K, Targher G, Chonchol M, Jalal DI. Association between nonalcoholic liver disease and chronic kidney disease: an ultrasound analysis from NHANES 1988–1994. Am J Nephrol 2012;36:466-71.ArticlePubMedPMCPDF
- 13. Jenks SJ, Conway BR, Hor TJ, Williamson RM, McLachlan S, Robertson C, et al. Hepatic steatosis and non-alcoholic fatty liver disease are not associated with decline in renal function in people with type 2 diabetes. Diabet Med 2014;31:1039-46.ArticlePubMed
- 14. Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 2014;11:e1001680.ArticlePubMedPMC
- 15. Targher G, Chonchol M, Bertolini L, Rodella S, Zenari L, Lippi G, et al. Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J Am Soc Nephrol 2008;19:1564-70.ArticlePubMedPMC
- 16. Targher G, Mantovani A, Pichiri I, Mingolla L, Cavalieri V, Mantovani W, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of chronic kidney disease in patients with type 1 diabetes. Diabetes Care 2014;37:1729-36.ArticlePubMedPDF
- 17. Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 2008;51:444-50.ArticlePubMedPDF
- 18. Targher G, Pichiri I, Zoppini G, Trombetta M, Bonora E. Increased prevalence of chronic kidney disease in patients with type 1 diabetes and non-alcoholic fatty liver. Diabet Med 2012;29:220-6.ArticlePubMed
- 19. Lombardi R, Airaghi L, Targher G, Serviddio G, Maffi G, Mantovani A, et al. Liver fibrosis by FibroScan independently of established cardiovascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int 2020;40:347-54.ArticlePubMedPDF
- 20. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12.ArticlePubMedPMC
- 21. Akinmokun A, Selby PL, Ramaiya K, Alberti KG. The short insulin tolerance test for determination of insulin sensitivity: a comparison with the euglycaemic clamp. Diabet Med 1992;9:432-7.ArticlePubMed
- 22. Kim SK, Choi YJ, Huh BW, Kim CS, Park SW, Lee EJ, et al. Ratio of waist-to-calf circumference and carotid atherosclerosis in Korean patients with type 2 diabetes. Diabetes Care 2011;34:2067-71.ArticlePubMedPMCPDF
- 23. Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Targher G, et al. Ultrasonographic fatty liver indicator detects mild steatosis and correlates with metabolic/histological parameters in various liver diseases. Metabolism 2017;72:57-65.ArticlePubMed
- 24. Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2014;20:475-85.ArticlePubMedPMC
- 25. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010;42:503-8.ArticlePubMed
- 26. Mantovani A, Zaza G, Byrne CD, Lonardo A, Zoppini G, Bonora E, et al. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: a systematic review and meta-analysis. Metabolism 2018;79:64-76.ArticlePubMed
- 27. Sinn DH, Kang D, Jang HR, Gu S, Cho SJ, Paik SW, et al. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: a cohort study. J Hepatol 2017;67:1274-80.ArticlePubMed
- 28. Vilar-Gomez E, Calzadilla-Bertot L, Friedman SL, Gra-Oramas B, Gonzalez-Fabian L, Villa-Jimenez O, et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2017;45:332-44.ArticlePubMedPDF
- 29. Li Y, Liu L, Wang B, Wang J, Chen D. Simple steatosis is a more relevant source of serum inflammatory markers than omental adipose tissue. Clin Res Hepatol Gastroenterol 2014;38:46-54.ArticlePubMed
- 30. Crasto C, Semba RD, Sun K, Ferrucci L. Serum fibroblast growth factor 21 is associated with renal function and chronic kidney disease in community-dwelling adults. J Am Geriatr Soc 2012;60:792-3.ArticlePubMedPMC
- 31. Byrne CD, Targher G. NAFLD as a driver of chronic kidney disease. J Hepatol 2020;72:785-801.ArticlePubMed
- 32. Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol 2010;5:2166-71.ArticlePubMedPMC
- 33. Schwabe RF, Tabas I, Pajvani UB. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology 2020;158:1913-28.ArticlePubMedPMC
- 34. Gu YY, Liu XS, Huang XR, Yu XQ, Lan HY. Diverse role of TGF-β in kidney disease. Front Cell Dev Biol 2020;8:123.ArticlePubMedPMC
- 35. Wong RJ, Tran T, Kaufman H, Niles J, Gish R. Increasing metabolic co-morbidities are associated with higher risk of advanced fibrosis in nonalcoholic steatohepatitis. PLoS One 2019;14:e0220612.ArticlePubMedPMC
- 36. Cheah MC, McCullough AJ, Goh GB. Current modalities of fibrosis assessment in non-alcoholic fatty liver disease. J Clin Transl Hepatol 2017;5:261-71.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Esteatosis hepática metabólica y nefropatía diabética: una llamada a la acción
Salvador Benlloch, Francesc Moncho, Jose Luis Górriz
Nefrología.2024; 44(2): 129. CrossRef - Longitudinal Outcomes Associated With Metabolic Dysfunction-Associated Steatotic Liver Disease: A Meta-analysis of 129 Studies
Kai En Chan, Elden Yen Hng Ong, Charlotte Hui Chung, Christen En Ya Ong, Benjamin Koh, Darren Jun Hao Tan, Wen Hui Lim, Jie Ning Yong, Jieling Xiao, Zhen Yu Wong, Nicholas Syn, Apichat Kaewdech, Margaret Teng, Jiong-Wei Wang, Nicholas Chew, Dan Yock Young
Clinical Gastroenterology and Hepatology.2024; 22(3): 488. CrossRef - Association of NAFLD/NASH, and MAFLD/MASLD with chronic kidney disease: an updated narrative review
Amedeo Lonardo
Metabolism and Target Organ Damage.2024;[Epub] CrossRef - Targeting metabolic-associated fatty liver disease in diabetic kidney disease: A call to action
Salvador Benlloch, Francesc Moncho, Jose Luis Górriz
Nefrología (English Edition).2024;[Epub] CrossRef - MAFLD and NAFLD in the prediction of incident chronic kidney disease
So Yoon Kwon, Jiyun Park, So Hee Park, You-Bin Lee, Gyuri Kim, Kyu Yeon Hur, Janghyun Koh, Jae Hwan Jee, Jae Hyeon Kim, Mira Kang, Sang-Man Jin
Scientific Reports.2023;[Epub] CrossRef - Associations of non-invasive indices of liver steatosis and fibrosis with progressive kidney impairment in adults with type 2 diabetes
Mei Chung Moh, Sharon Li Ting Pek, Kenny Ching Pan Sze, Serena Low, Tavintharan Subramaniam, Keven Ang, Wern Ee Tang, Simon Biing Ming Lee, Chee Fang Sum, Su Chi Lim
Acta Diabetologica.2023; 60(6): 827. CrossRef - Pancreatic beta-cell specific BAG3 knockout results in chronic hyperinsulinemia inducing insulin resistance
Verena Damiani, Alessia Lamolinara, Ilaria Cicalini, Maria Concetta Cufaro, Francesco Del Pizzo, Federica Di Marco, Piero Del Boccio, Beatrice Dufrusine, Michael Hahne, Rossano Lattanzio, Damiana Pieragostino, Manuela Iezzi, Massimo Federici, Maria Cateri
Molecular Metabolism.2023; 74: 101752. CrossRef - Utility of non-invasive liver fibrosis markers to predict the incidence of chronic kidney disease (CKD): A systematic review, meta-analysis, and meta-regression
Rudi Supriyadi, Theo Audi Yanto, Timotius Ivan Hariyanto, Ketut Suastika
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(8): 102814. CrossRef - Significance of Diabetic Kidney Disease Biomarkers in Predicting Metabolic-Associated Fatty Liver Disease
Jaehyun Bae, Byung-Wan Lee
Biomedicines.2023; 11(7): 1928. CrossRef - Hepatic Fibrosis Evaluated in Patients with Type 2 Diabetes with and without Chronic Kidney Disease
Therese Adrian, Mads Hornum, Filip Krag Knop, Karl Bang Christensen, Thomas Almdal, Peter Rossing, Lisa Í Lídaa, Niels Søndergaard Heinrich, Vincent Oltman Boer, Anouk Marsman, Esben Thade Petersen, Hartwig Roman Siebner, Bo Feldt-Rasmussen
Nephron.2023; 147(11): 673. CrossRef - Clinical Interest of Serum Alpha-2 Macroglobulin, Apolipoprotein A1, and Haptoglobin in Patients with Non-Alcoholic Fatty Liver Disease, with and without Type 2 Diabetes, before or during COVID-19
Olivier Deckmyn, Thierry Poynard, Pierre Bedossa, Valérie Paradis, Valentina Peta, Raluca Pais, Vlad Ratziu, Dominique Thabut, Angelique Brzustowski, Jean-François Gautier, Patrice Cacoub, Dominique Valla
Biomedicines.2022; 10(3): 699. CrossRef - Fibrosis Risk in Nonalcoholic Fatty Liver Disease Is Related to Chronic Kidney Disease in Older Type 2 Diabetes Patients
Yifan Sun, Liang Hong, Zhe Huang, Lihong Wang, Yanqin Xiong, Shuhang Zong, Rui Zhang, Jun Liu, Shufei Zang
The Journal of Clinical Endocrinology & Metabolism.2022; 107(9): e3661. CrossRef - Beyond Liver Disease: Non-Alcoholic Fatty Liver Disease and Advanced Liver Fibrosis in Kidney Disease
Eugene Han
Diabetes & Metabolism Journal.2022; 46(4): 564. CrossRef - A higher FIB‐4 index is associated with an increased incidence of renal failure in the general population
Eva Maria Schleicher, Simon Johannes Gairing, Peter Robert Galle, Julia Weinmann‐Menke, Jörn M. Schattenberg, Karel Kostev, Christian Labenz
Hepatology Communications.2022; 6(12): 3505. CrossRef - Advanced Liver Fibrosis Is Associated with Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease (Diabetes Metab J 2022;46:630-9)
Ji Hye Huh
Diabetes & Metabolism Journal.2022; 46(6): 953. CrossRef - Advanced Liver Fibrosis Is Associated with Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease (Diabetes Metab J 2022;46:630-9)
Da Hea Seo, So Hun Kim
Diabetes & Metabolism Journal.2022; 46(6): 956. CrossRef
- Figure
- Related articles
-
- Risk Prediction and Management of Chronic Kidney Disease in People Living with Type 2 Diabetes Mellitus
- Glycemic Control and Adverse Clinical Outcomes in Patients with Chronic Kidney Disease and Type 2 Diabetes Mellitus: Results from KNOW-CKD
- Advanced Liver Fibrosis Is Associated with Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease (Diabetes Metab J 2022;46:630-9)
- Advanced Liver Fibrosis Is Associated with Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease (Diabetes Metab J 2022;46:630-9)
- Safety and Effectiveness of Empagliflozin in Korean Patients with Type 2 Diabetes Mellitus: Results from a Nationwide Post-Marketing Surveillance

 KDA
KDA
 PubReader
PubReader ePub Link
ePub Link Cite
Cite