
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 37(5); 2013 > Article
-
ReviewPathophysiology SIRT1 in Type 2 Diabetes: Mechanisms and Therapeutic Potential
- Munehiro Kitada, Daisuke Koya
-
Diabetes & Metabolism Journal 2013;37(5):315-325.
DOI: https://doi.org/10.4093/dmj.2013.37.5.315
Published online: October 17, 2013
Division of Diabetology and Endocrinology, Kanazawa Medical University, Kahoku, Japan.
- Corresponding author: Daisuke Koya. Division of Diabetology and Endocrinology, Kanazawa Medical University, 1-1 Daigaku, Uchinada, Kahoku 920-0293, Japan. koya0516@kanazawa-med.ac.jp
Copyright © 2013 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- The prevalence of type 2 diabetes mellitus (T2DM) has been increasing worldwide. Therefore, a novel therapeutic strategy by which to prevent T2DM is urgently required. Calorie restriction (CR) can retard the aging processes, and delay the onset of numerous age-related diseases including diabetes. Metabolic CR mimetics may be therefore included as novel therapeutic targets for T2DM. Sirtuin 1 (SIRT1), a NAD+-dependent histone deacetylase that is induced by CR, is closely associated with lifespan elongation under CR. SIRT1 regulates glucose/lipid metabolism through its deacetylase activity on many substrates. SIRT1 in pancreatic β-cells positively regulates insulin secretion and protects cells from oxidative stress and inflammation, and has positive roles in the metabolic pathway via the modulation in insulin signaling. SIRT1 also regulates adiponectin secretion, inflammation, glucose production, oxidative stress, mitochondrial function, and circadian rhythms. Several SIRT1 activators, including resveratrol have been demonstrated to have beneficial effects on glucose homeostasis and insulin sensitivity in animal models of insulin resistance. Therefore, SIRT1 may be a novel therapeutic target for the prevention of T2DM, implicating with CR. In this review, we summarize current understanding of the biological functions of SIRT1 and discuss its potential as a promising therapeutic target for T2DM.
- The incidence and prevalence of diabetes mellitus have significantly increased worldwide in recent decades, primarily due to the increase in type 2 diabetes mellitus (T2DM). Long-term diabetes results in vascular changes and dysfunction, and diabetic vascular complications are the major cause of morbidity and mortality in patients with diabetes. It is not a simple matter to strictly control blood glucose levels for long periods of time, even given the many antidiabetic medications that are clinically available. Therefore, the development of improved additional treatments and novel prevention strategies for T2DM is a matter of great urgency.
- Aging is implicated in metabolic diseases, including diabetes; therefore, aging is recognized as a risk factor for the initiation and the development of T2DM. Calorie restriction (CR) without malnutrition promotes longevity and slows aging. Numerous studies have revealed that CR retards aging or extends the lifespans of yeast, worms, flies, and rodents. Colman et al. [1] also reported that 30% CR delayed the onset of numerous age-associated pathologies, including diabetes, cancer, cardiovascular disease and brain atrophy, and decreased mortality in rhesus monkeys. Moreover, Fontana et al. [2] reported that CR for an average of 6 years improved metabolism in humans, as was indicated by levels of serum insulin, cholesterol, C-reactive protein (CRP) and tumor necrosis factor (TNF)-α as well as by carotid intima media thickness. This group also observed that long-term CR ameliorated the decline in left ventricular diastolic function and decreased levels of serum tumor growth factor-β1, TNF-α, and high-sensitivity CRP [3]. Thus, CR has a variety of beneficial effects with respect to lifespan extension and delays the onset of age-related diseases, such as cardiovascular diseases, neurodegenerative disorders, and diabetes. CR is defined as the restriction of food intake without malnutrition in organisms that are normally fed ad libitum, and it is accepted as the only established antiaging experimental paradigm.
- As one of the molecules through which CR improves lifespan extension or delays age-related diseases, initial studies of aging in yeast identified silent information regulator 2 (Sir2), which is a NAD+-dependent deacetylase. Homologues of Sir2 in higher eukaryotic organisms are referred to as sirtuins. SIRT1, the sirtuin that is most closely related to Sir2, is one of seven sirtuins in mammals. The beneficial effects of CR involve the function of SIRT1, which is induced by CR in various tissues. The significance of SIRT1 on the effects of CR has been demonstrated using genetically altered mice. Bordone et al. [4] reported that Sirt1 transgenic mice exhibited a CR-like phenotype, exhibiting reduced levels of blood cholesterol, adipokines, insulin, and fasting glucose and greater glucose tolerance than control mice. However, Sirt1 deficiency in mice fails to extend lifespan under CR [5]. Additionally, a 25% reduction in calorie intake for 6 months in nonobese young adults led to the upregulation of SIRT1 and peroxisome proliferator activated receptor (PPAR)-γ coactivator-1α (PGC-1α) in the skeletal muscle. This effect was accompanied by an increase in mitochondrial function and a decrease in visceral fat mass, insulin resistance, body temperature, metabolic rate, and levels of oxidative stress [6]. Thus, SIRT1 is an important regulator of energy metabolism, and appears to be required for a normal response to CR. Furthermore, recent reports demonstrate that SIRT1 is downregulated in several cells and tissues in insulin-resistant or glucose intolerance states [7-9]. Therefore, under excess energy intake, decreased SIRT1 activity may contribute to the development of obesity-related conditions, including insulin resistance and T2DM. Diet therapy, including CR, is generally necessary for patients with T2DM; however, it is not a simple matter for patients to strictly control their diet over the long term. Therefore, SIRT1 activation, as a CR mimetic, may be a candidate therapeutic target for T2DM.
INTRODUCTION
- SIRT1 functions as class III histone deacetylases, binding to NAD+ and acetyllysine within protein targets and generating lysine, 2'-O-acetyl-ADP-ribose, and nicotinamide as enzymatic products. Nicotinamide acts as a negative-feedback inhibitor of SIRT1 (Fig. 1).
- SIRT1 regulates a wide variety of cellular functions, such as metabolism related to glucose-lipid metabolism, mitochondrial biogenesis, inflammation, autophagy, and circadian rhythms, and others including, stress resistance, apoptosis and chromatin silencing (Table 1) [10]. SIRT1 can act on more than a dozen nonhistone proteins, including transcription factors, transcriptional coregulatory proteins, and histones. SIRT1 participates in the control of systemic metabolism via the regulation of glucose and lipid homeostasis by deacetylating various targets. PGC-1α is an important factor in mitochondrial biogenesis and function and is regulated by an acetylation/deacetylation reaction. The transcription factor forkhead box O1 (FOXO1) is involved in the control of glucose-lipid metabolism and stress resistance. In addition, SIRT1 also regulates components of the circadian clock, such as brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1) and period 2 (PER2). SIRT1 is associated with lipid metabolism through the activation of nuclear receptors, including PPAR-α, liver X receptor (LXR), and farnesoid X receptor (FXR) and via the negative regulation of sterol regulatory element binding protein (SREBP). Furthermore, SIRT1 deacetylates transcription factors, such as p53, poly-ADP-ribose polymerase-1, hypoxia inducible factors (HIFs)-1α and HIF-2α, nuclear factor (NF)-κB, autophagy-related gene (Atg) 5, Atg7, and light chain 3. These functions mediate stress resistance, apoptosis, hypoxia, inflammatory signaling, and autophagy as physiological responses to environmental toxicity. Thus, the SIRT1 activation may lead to the induction of gene silencing, reduced apoptosis, enhanced mitochondrial biogenesis, the inhibition of inflammation, the regulation of glucose and lipid metabolism and circadian rhythms, the induction of autophagy and adaptations to cellular stress.
SIRT1 AND ITS BIOLOGICAL FUNCTION
- SIRT1 may participate in the control of glucose homeostasis through the following mechanisms: regulating insulin secretion [11] and protecting pancreatic β-cells [12]; improving insulin resistance via the modulation of postinsulin receptor signaling; decreasing inflammation, lipid mobilization, and adiponectin excretion [13]; controlling fatty acid oxidation and mitochondrial biogenesis [14]; and regulating hepatic glucose production and circadian rhythms, skeletal muscle, adipose tissue, monocytes/macrophages, and the liver (Table 2). Therefore, SIRT1 is a promising pharmacological therapeutic target for the treatment of insulin-resistance and subsequent T2DM [15].
- Several studies have suggested that SIRT1 participates in the regulation of insulin secretion from pancreatic β-cells. The SIRT1 overexpression in β-cells enhances adenosine triphosphate (ATP) production by repressing uncoupling protein (UCP) 2. This process mediates the uncoupling of ATP synthesis from glucose, and elevated ATP levels lead to cell membrane depolarization and Ca2+-dependent insulin exocytosis. β-Cells in SIRT1-deficient mice, however, produce less ATP in response to glucose than do normal mice. By deacetylating FOXO1, SIRT1 also promotes the activation and transcription of NeuroD and MafA, preserving insulin production and promoting β-cell survival in vivo [11]. Additionally, Lee et al. [12] demonstrated that SIRT1 protects β-cells against various toxic stresses, such as oxidative stress and cytokines, by suppressing NF-κB signaling. In β-cell-specific SIRT1 overexpression (BESTO) mice, increased SIRT1 levels in pancreatic β-cells improve glucose tolerance and enhance insulin secretion in response to glucose [16]. Moreover, SIRT1 activity decreases with age due to decreased systemic NAD+ biosynthesis, resulting in the failure of glucose-sensitive insulin secretion in β-cells. However, the administration of nicotinamide mononucleotide, a metabolite that is important for the maintenance of normal NAD+ biosynthesis, restores glucose-sensitive insulin secretion and improves glucose tolerance in aged BESTO mice [17]. These findings indicate that SIRT1 modulates glucose-sensing ATP production and insulin secretion from β-cells through UCP2, FOXO1, and NAD+ metabolism, resulting in protective effects against various toxic stresses through NF-κB pathway activation.
- SIRT1 can directly interact with the insulin signaling pathway through several mechanisms. SIRT1 represses the expression of tyrosine phosphatase1 B, which negatively regulates insulin signaling in skeletal muscle, primarily through dephosphorylation of tyrosine residues on the insulin receptor (IR) and insulin receptor substrate (IRS)-1 [18]. Zhang [19] reported that SIRT1 regulates the insulin-induced tyrosine phosphorylation of IRS-2 through its deacetylation, which affects a crucial step in the insulin signaling pathway. In brief, the insulin-induced tyrosine phosphorylation of the IR and the activation of SIRT1 deacetylase were suggested to be separate events in the insulin signaling pathway. Although IRS-2 is acetylated at the basal state, insulin treatment leads to the tyrosine phosphorylation of the IR, which further recruits IRSs, including IRS-1 and IRS-2, to its kinase domain. The acetylated lysine residues in IRS-2 prevent IR kinase from further phosphorylating the tyrosine residues in IRS-2. Continued phosphorylation of the tyrosine residues in IRS-2 requires the removal of its acetylated lysine residues by insulin-activated SIRT1, and phosphorylated IRS-2 can then serve as an adaptor protein to further transmit insulin signaling to downstream targets, such as Akt [19]. Moreover, Frojdo et al. [9] demonstrated that SIRT1 protein expression was decreased in muscle biopsies and primary myotubes that were derived from subjects with T2DM and that this effect was likely due to posttranscriptional modifications, as no differences in SIRT1 mRNA levels were observed between the controls and type 2 diabetic patients. Moreover, SIRT1 interacts in an insulin-independent manner with the phosphoinositide 3-kinase (PI3K) adapter subunit p85 and modulates insulin signaling at physiological insulin concentrations in skeletal muscle cells [9]. PI3K interacts with IRS following insulin-stimulated tyrosine phosphorylation of IR; insulin signaling can then continue to activate downstream molecules, such as Akt. Therefore, SIRT1 may positively regulate insulin signaling by interacting with PI3K. In addition, the SIRT1 activator resveratrol protects muscle cells, including human primary myotubes, from TNF-α or prolonged hyperinsulinemia-induced insulin resistance. SIRT1 protein can be detected in both nuclear and cytosolic fractions by cell fractionation, and interestingly, nuclear-associated SIRT1 interacts with cytoplasmic proteins, such as IRS-2.
- Chronic low grade tissue inflammation is an important etiologic component of insulin resistance and T2DM. Elevated levels of proinflammatory cytokines, such as TNF-α, IL-6, and CRP, in the blood have been detected in individuals with insulin resistance and T2DM. The activation of monocytes in the circulation and adipose tissue has been demonstrated to lead to the release of various inflammatory mediators. Additionally, it has been demonstrated that macrophages residing in adipose tissue may also be a source of inflammatory factors and that these cells may modulate the secretory activity of adipocytes. Tissue macrophages, which are derived from blood monocytes play a central role in both orchestrating and initiating obesity-related tissue inflammatory responses. Moreover, monocytes/macrophages and adipose tissue have reported to exhibit significantly increased binding to NF-κB, the key proinflammatory transcription factor, and an increased levels of intranuclear expression of p65 (Rel A), the major protein component of NF-κB. Thus, the suppression of inflammatory cytokines overproduction in monocytes/macrophages and adipocytes may improve insulin resistance and T2DM. Decreased SIRT1 expression levels in circulating monocytes are correlated with metabolic syndrome, insulin resistance, and glucose intolerance in humans [7]. Moreover, Gillum et al. [8] reported that SIRT1 expression was reduced in adipose tissues of obese males. In addition, mRNA expression of CD14, a macrophage marker, in adipose tissue is negatively correlated with SIRT1 expression. These data indicate that SIRT1 may contribute to the regulation of inflammation in monocytes/macrophages and adipose tissue in humans [8]. Schug et al. [20] also demonstrated that myeloid cell-specific SIRT1 knockout mice that were challenged with a high fat diet displayed high levels of activated macrophages in the liver and adipose tissues, thereby predisposing these animals to the development of systemic insulin resistance and metabolic derangement. SIRT1 physically interacts with the p65 subunit of NF-κB and inhibits transcription by deacetylating p65 at lysine 310, leading to the suppression of inflammatory processes. Yoshizaki et al. [21] provided direct evidence that SIRT1 activation reduced the TNF-α-induced inflammatory response, potentially via the deacetylation of NF-κB (p65) in insulin-resistant adipocytes. Moreover, these authors reported that SIRT1 knockdown in 3T3-L1 adipocytes increased NF-κB (p65) acetylation and enhanced NF-κB binding to target inflammation-related genes promoters [21]. In addition, Yoshizaki et al. [22] reported that SIRT1 represses the activity of the IκB kinase (IKK)-NF-κB signaling pathway, inflammation-related gene expression, and the release of TNF-α following lipopolysaccharide stimulation in macrophages. These authors reported that the pharmacological SIRT1 activator SRT1720 or resveratrol induced various anti-inflammatory activities [22]. Furthermore, the treatment of obese and insulin-resistant Zucker fatty rats with another SIRT1 activator, SRT2379, led to improved glucose tolerance, enhanced systemic insulin sensitivity, and the normalization of tissue markers of inflammation [22]. Additionally, our recent report provided another mechanism with which to explain how SIRT1 inactivation induces inflammation in THP-1 cells. Specifically, SIRT1 inhibition may activate the NF-κB signaling pathway through the phosphorylation of NF-κB (p65) via the dysregulation of autophagy, resulting in the cellular accumulation of p62/Sqstm1 [23]. Moreover, the nutrient-sensing pathway regulates autophagy and involves SIRT1, mammalian target of rapamycin (mTOR) and 5' adenosine monophosphate (AMP)-activated kinase (AMPK). Notably, SIRT1 inactivation resulted in increased mTOR pathway activation and reduced AMPK activation, leading to impaired autophagy [23]. Thus, SIRT1 may attenuate the inflammatory reaction in adipose tissues and monocytes/macrophages and thereby improve insulin resistance and T2DM.
- Adipocytes play critical roles in the development of insulin resistance and T2DM given that they can store excess saturated lipids and produce adipokines. PPAR-γ is an essential molecule for the modulation of fatty acid storage and glucose metabolism, and this factor is involved in adipose tissue differentiation. In mature white fat cells, PPAR-γ regulates the induction of genes that are involved in free fatty acid (FFA) uptake and triglyceride synthesis, thereby increasing the lipid storage capacity of the cell [13]. SIRT1 binds to PPAR-γ by docking to the nuclear receptor corepressor and silencing the mediator of retinoid and thyroid hormone receptors, effects that represses the transcription-activating effects of PPAR-γ [13]. Furthermore, SIRT1 overexpression was observed to lead to decreased fat storage and increased lipolysis, resulting in fat mobilization in response to food limitation [13], whereas SIRT1-null mice exhibited a significant reduction in body weight. Additionally, in the adipose tissue of those SIRT1-null mice, the average size of the adipocytes was smaller, the content of the extracellular matrix was lower, adiponectin and leptin were expressed at 60% of the normal level, and adipocyte differentiation was reduced [24]. Moreover, a recent report demonstrated that SIRT1 promotes browning of white fat. SIRT1 deacetylates ligand-bound PPAR-γ on Lys268 and Lys293; therefore, SIRT1 and PPAR-γ coordinately induce the browning of white adipose tissue [25]. These data indicate that SIRT1-dependent PPAR-γ deacetylation regulates energy homeostasis, promoting energy expenditure over energy storage. Therefore, the combination of thiazolidinediones with SIRT1 activator has potential as a therapy for obesity. Adiponectin exerts an antidiabetic effect, and plasma adiponectin levels are decreased in the contexts of obesity, insulin resistance, and T2DM. The administration of adiponectin has been demonstrated to induce glucose-lowering effects and to improve insulin resistance in mice [26]. Moreover, adiponectin-deficient mice exhibit insulin resistance and diabetes [27]. The mechanisms by which adiponectin exerts its insulin-sensitizing effects may be mediated by an increase in fatty acid oxidation via the activation of AMPK and PPAR-α. Additionally, SIRT1 regulates adiponectin expression in adipocytes and FOXO1 forms a transcriptional complex at the mouse adiponectin promoter with CCAAT/enhancer-binding protein α (C/EBPα) [28]. Thus, SIRT1 deacetylates FOXO1 and enhances its interaction with C/EBPα, resulting in the enhanced transcription of the gene that encodes adiponectin in adipocytes. Moreover, a study of muscle adiponectin receptor (adipoR) 1KO mice demonstrated that this protein has a crucial role in the physiological and pathophysiological significance of adiponectin in muscle cells and is involved in the regulation of Ca2+ signaling as well as PGC-1α expression and activation. Adiponectin activates AMPK by biding to adipoR1, thereby activating SIRT1 and deacetylating PGC-1α to improve mitochondrial function, oxidative stress, glucose and lipid metabolism, and exercise endurance [29].
- SIRT1 can affect glucose-lipid metabolism and insulin resistance through the modulation of mitochondrial function. The maintenance of energy and nutrient homeostasis during nutrient deprivation is accomplished through an increase in mitochondrial fatty acid oxidation in skeletal muscle. Previous studies have demonstrated a reduced rate of mitochondrial oxidative phosphorylation (OXPHOS) activity and increased intramyocellular lipid accumulation in the skeletal muscle of insulin-resistant patients with type 2 diabetes and elderly individuals [30]. Specifically, these data indicate that defects in mitochondrial function may play an important role in T2DM pathogenesis. An important component that drives this cellular oxidative process in mitochondria is the transcriptional coactivator PGC-1α. PGC-1α activation in skeletal muscle leads to efficient β-oxidation of fatty acids, which is coupled to mitochondrial OXPHOS. In addition, PGC-1α maintains higher numbers of active mitochondria and OXPHOS protein, the levels of which are decreased in T2DM. Through PGC-1α regulation, SIRT1 modulates mitochondrial function and metabolic homoeostasis, increases the consumption of oxygen in muscle fibers and induces the expression of OXPHOS genes and mitochondrial biogenesis. Remarkably, the PGC-1α-induced upregulation of genes that regulate mitochondrial fatty acid utilization was largely prevented by SIRT1 knockdown [14]. Furthermore, SIRT1 can regulate PPAR-α activation through PGC-1α deacetylation, leading to the increased fatty acid oxidation [31]. Thus, SIRT1 activation may improve insulin resistance via accelerated fatty acid oxidation and mitochondrial biogenesis in skeletal muscle. In addition to the effect of increased lipid utilization via PGC-1α-mediated mitochondrial biogenesis, PGC-1α markedly upregulates glucose transporter 4 (GLUT4) expression and glucose transport activity in murine C2C12 myotubes [32]. The effects of PGC-1α on the activation of GLUT4 gene expression are reflected in the increased ability of myocytes to transport glucose, suggesting that the SIRT1-regulated activation of PGC-1α influences insulin sensitization.
- The liver plays a central role in glucose and lipid metabolism in response to nutritional and hormonal signals. In a fasted state, the induction of hepatic glucose output and fatty acid oxidation is essential to sustain energetic balance. The production of glucose by the liver is controlled through a complex network of transcriptional regulators. During the early stage of fasting, glucagon induces cyclic AMP (cAMP) response element-binding (CREB) and CREB-regulated transcription coactivator 2 (CRTC2) to drive the expression of gluconeogenesis-related genes that supply the body with the necessary glucose [33]. At the late stage of fasting, SIRT1 is activated and deacetylates CRTC2 to reduce the effects of glucagon [34]. Moreover, at that time, SIRT1 can activate PGC-1α and FOXO1 through a deacetylation reaction, resulting in the induction of gluconeogenesis-related genes. Thus, SIRT1 participates in the regulation of the metabolic switch that controls the shift from the early to the late phase of gluconeogenesis during fasting to maintain glucose homeostasis. Conversely, various reports using animal models have indicated that SIRT1 may have an antidiabetic function. Transgenic mice with moderate SIRT1 overexpression exhibited improved glucose tolerance due to reduced glucose output from the liver [35]. Additionally, Wang et al. [36] also demonstrated that SIRT1 negatively regulates gluconeogenesis. In liver-specific SIRT1-deficient mice, the reduced expression of Rictor, which is a key component of the mTORC2 complex, impaired the Akt-S473 phosphorylation, caused FOXO1-S253 hypophosphorylation, and increased G6pase and Pepck expression to establish chronic hyperglycemia [36]. However, other liver-specific SIRT1 knockout mice exhibit normal glucose levels under both fasting and fed conditions [37]. Moreover, acute SIRT1 knockdown in the mouse liver using an adenovirus system [38] or SIRT1 knockdown in the livers of type 2 diabetic rats using antisense oligonucleotides decreased basal hepatic glucose production and increased hepatic insulin responsiveness to glucose [39]. SIRT1 has also been demonstrated to regulate gluconeogenesis through the deacetylation of signal transducers and activators of transcription (STAT) 3 [40]. STAT3 suppresses gluconeogenesis by inhibiting the transcriptional activity of gluconeogenesis-related gene expression. SIRT1 deacetylates STAT3, resulting in a decrease in STAT3 activity and the subsequent inhibition of gluconeogenesis. Therefore, SIRT1 induces glucose output from the liver in response to fasting via the deacetylation, and thereby inhibition, of STAT3. These results indicate that SIRT1 has a complex role in the regulation of hepatic glucose metabolism under different conditions through the alteration in the expression of gluconeogenesis genes and the modulation of CTRC2, PGC-α, FOXO1, and STAT3 activity.
- Dyslipidemia often coincides with T2DM. During fasting or energy limitation, the liver increases lipid utilization and decreases lipid and cholesterol synthesis. Reduced fatty acid oxidation causes hepatic steatosis, which is correlated with insulin resistance. SIRT1 enhances mitochondrial fatty acid oxidation in response to fasting by activating PPAR-α and PGC-1α in the liver. Moreover, SIRT1 regulates SREBP and LXR, both of which are involved in lipid synthesis in the liver: SIRT1 deacetylates and inhibits SREBP-1C activity [41], resulting in decreased lipid synthesis, and deacetylates and positively regulates LXR, contributing to reverse cholesterol transport from peripheral tissues [42]. SIRT1 also activates FXR, which is involved in cholesterol catabolism [43]. In liver-specific SIRT1 knockout mice, the induction of fatty acid oxidation through PPAR-α and PGC-1α was reported to decrease, resulting in increased levels of hepatic FFAs and hepatic steatosis [38,44]. In addition, high fat diet-induced hepatic steatosis was improved in mice with overexpressed SIRT1 [45] and treatment with SIRT1 activators such as resveratrol [41]. Interestingly, recent reports have also indicated that the treatment of obese humans with resveratrol attenuates hepatic fat content and improves insulin resistance [46].
- Oxidative stress impairs the insulin signaling pathway and leads to the onset and progression of insulin resistance in T2DM. In hyperglycemia, other metabolites, including FFA and several cytokines, such as TNF-α, induce the overproduction of reactive oxygen species (ROS) by the mitochondria, which are a primary source of ROS. ROS trigger the activation of serine/threonine kinases, such as apoptosis signal-regulating kinase 1, c-jun N-terminal kinase, and IKK, which in turn increase the serine phosphorylation of IRS-1 and decrease the tyrosine phosphorylation of IRS-1. This effect results in insulin resistance and inflammation (oxidative stress linked to inflammation). Thus, reduced mitochondrial oxidative capacity can cause insulin resistance through oxidative stress. PGC-1α deacetylation by SIRT1 mediates mitochondrial biogenesis in addition to the overexpression of antioxidative enzymes, such as Mn-SOD [47], thereby reducing oxidative stress caused by the impaired mitochondria. Moreover, FOXO3a is deacetylated by SIRT1 and translocated to the nucleus, resulting in the upregulated catalase and protection against oxidative stress [48].
- The circadian clock, which produces physiological and behavioral rhythms, drives cycles of energy storage and utilization in the anticipation of changes during the day and night, and recent studies have revealed an association between the circadian clock and cellular metabolism. The transcription factors CLOCK and BMAL1 pay a central role in the regulation of circadian gene expression by binding to E-box elements within the promoters of clock-controlled genes (CCGs). Turek et al. [49] demonstrated that homozygous Clock mutant mice exhibited a greatly attenuated diurnal feeding rhythm in addition to hyperphagia and obesity. Moreover, these mice developed a metabolic syndrome that was associated with hyperglycemia, hypoinsulinemia, and hepatic steatosis [49]. Additionally, a high fat diet may disrupt behavioral and molecular circadian rhythms by altering the expression and cycling of clock genes, nuclear receptors and CCGs in the hypothalamus, fat and liver. These findings indicate that nutrient excess may affect the onset and progression of obesity-related diseases, such as diabetes [50]. Moreover, SIRT1 is a key modulator of the circadian clock machinery, and SIRT1 expression or activity both oscillates in a circadian manner and is associated with circadian oscillations in NAD+ levels [51]. The CLOCK-BMAL1 complex interacts with SIRT1 and binds to the promoters of circadian genes, including PER, cryptochrome (CRY), and nicotinamide phosphoribosyltransferase, which encodes the rate-limiting enzyme in NAD+ biosynthesis. Recent reports have demonstrated that SIRT1 participates in the regulation of circadian rhythms via the deacetylation of BMAL1, PER2, and histones H3K9 and H3K14 [51,52]. Acetylated BMAL1 recruits CRY [53], a negative regulator of circadian-controlled gene expression, and promotes the acetylation of PER2, a negative regulator of CLOCK-BMAL1 transcription, thereby enhancing its stability [52]. Thus, SIRT1 links cellular metabolism to the circadian clock in a feedback loop.
SIRT1 AS A THERAPEUTIC PERSPECTIVE IN T2DM
- Resveratrol (3,5,4'-trihydroxystilbene), a natural polyphenolic compound that is found in grapes and red wine, is a SIRT1 activator. Numerous reports demonstrate the effects of resveratrol on the improvement of metabolic disorders in ob/ob, db/db, and high fat diet-induced obese mice or Zucker fa/fa rats [15,54]. In addition, resveratrol has exhibited beneficial effects on the longevity and metabolic abnormalities in high fat diet-induced obese mice; however, this compound exhibited no effect on lifespan extension in standard diet-fed mice. In humans, Timmers et al. [46] reported that the administration of oral resveratrol (150 mg/day) to obese male patients for 30 days resulted in CR-like effects, such as improved insulin sensitivity, triglyceride levels, energy expenditure, hepatic lipid accumulation, and the activation of the AMPK/SIRT1 pathway in skeletal muscle. Brasnyo et al. [55] also demonstrated that treatment with resveratrol (10 mg/day) in T2DM patients improves insulin sensitivity and oxidative stress, leading to more efficient insulin signaling via the Akt pathway. However, other recent reports indicate that resveratrol has no effects on metabolism, including insulin resistance. Yoshino et al. [56] demonstrated that oral resveratrol (75 mg/day) supplementation in nonobese and postmenopausal women with normal glucose tolerance does not improve metabolic function, such as insulin sensitivity. Poulsen et al. [57] also reported that high dose of resveratrol (500 mg/day) supplementation in obese men has no effects on the insulin sensitivity, turnover and oxidation of glucose. Thus, the efficacy of resveratrol for metabolism is controversial in humans, and further studies are required.
- Resveratrol is not a SIRT1-specific activator, and the mechanism by which resveratrol activates SIRT1 remains unclear. Although resveratrol originally directly can activate SIRT1 allosterically [58], AMPK is required upstream for the activation of SIRT1 by resveratrol. Additionally, Park et al. [59] reported that resveratrol activates SIRT1 through the activation of AMPK via the inhibition of phosphodiesterase 4 and the elevation of cAMP in cells, thereby providing a novel mechanism by which to explain SIRT1 activation by resveratrol. A recent study reported by Price et al. [60] also demonstrated a direct link between SIRT1 and the metabolic benefits of resveratrol. These authors reported that a moderate dose of resveratrol first activated SIRT1 and then induced the deacetylation of liver kinase B 1 and AMPK activation, leading to increased mitochondrial biogenesis and function [60]. Moreover, a high dose of resveratrol may directly activate AMPK, independently of SIRT1.
- Synthetic compounds, such as SRT1720 and SRT2379, which are structurally distinct from resveratrol but have potent SIRT1-activating power in vitro have been synthesized by Sirtris Pharmaceuticals. Among these compounds, the treatment of high fat diet-induced obese [21] and ob/ob mice with SRT1720 resulted in an improvement of insulin sensitivity, lower plasma glucose, and increased mitochondrial capacity [54]. In addition, in Zucker fa/fa rats, SRT1720 treatment improved whole glucose homeostasis as evaluated using hyperinsulinemic-euglycemic clamp studies, as well as insulin sensitivity in adipose tissue, skeletal muscle, and liver. Furthermore, Yoshizaki et al. [21,22] also demonstrated the efficacy of the SIRT1 activator SRT2379 against insulin resistance in high fat diet induced obese mice. These authors reported that this effect was related to reduced inflammation in adipocytes and macrophages.
SIRT1 ACTIVATING COMPOUNDS
- Over the last decade, our understanding of SIRT1 has expanded from its initial characterization as a single NAD+-dependent class III histone deacetylase that is responsible for longevity in yeast and which is associated with CR. Specifically, it has been found that SIRT1 deacetylates not only histones but also many transcriptional regulators and proteins, thereby modulating diverse biological processes. SIRT1 also may exert antidiabetic effects via the modulation of insulin secretion and improvement of insulin resistance via its regulatory effects on insulin signaling, inflammation, mitochondrial function, and circadian rhythms. Therefore, SIRT1 may be a novel therapeutic target for T2DM.
CONCLUSIONS
-
Acknowledgements
- This study was supported by a grant from Novo Nordisk Pharma, a grant-in-aid for scientific research (C) (24591218), a grant for promoted research from Kanazawa Medical University (S2012-4) to Munehiro Kitada and specially promoted research from Kanazawa Medical University (SR2012-06) and the 4th annual research award grant of Japanese Society of Anti-Aging Medicine to Daisuke Koya.
ACKNOWLEDGMENTS
- 1. Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009;325:201-204. ArticlePubMedPMC
- 2. Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A 2004;101:6659-6663. ArticlePubMedPMC
- 3. Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol 2006;47:398-402. ArticlePubMed
- 4. Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 2007;6:759-767. ArticlePubMed
- 5. Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, Xuan J, Evans M, Harper ME, McBurney MW. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One 2008;3:e1759ArticlePubMedPMC
- 6. Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. CALERIE Pennington Team. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med 2007;4:e76ArticlePubMedPMC
- 7. de Kreutzenberg SV, Ceolotto G, Papparella I, Bortoluzzi A, Semplicini A, Dalla Man C, Cobelli C, Fadini GP, Avogaro A. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes 2010;59:1006-1015. ArticlePubMedPMCPDF
- 8. Gillum MP, Kotas ME, Erion DM, Kursawe R, Chatterjee P, Nead KT, Muise ES, Hsiao JJ, Frederick DW, Yonemitsu S, Banks AS, Qiang L, Bhanot S, Olefsky JM, Sears DD, Caprio S, Shulman GI. SirT1 regulates adipose tissue inflammation. Diabetes 2011;60:3235-3245. ArticlePubMedPMCPDF
- 9. Frojdo S, Durand C, Molin L, Carey AL, El-Osta A, Kingwell BA, Febbraio MA, Solari F, Vidal H, Pirola L. Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by SIRT1. Mol Cell Endocrinol 2011;335:166-176. ArticlePubMed
- 10. Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, Koya D. Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clin Sci (Lond) 2013;124:153-164. ArticlePubMedPDF
- 11. Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol 2006;4:e31ArticlePubMed
- 12. Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, Park JW, Kwon KB, Park BH. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes 2009;58:344-351. PubMedPMC
- 13. Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004;429:771-776. ArticlePubMedPMCPDF
- 14. Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 2007;26:1913-1923. ArticlePubMedPMC
- 15. Kitada M, Kume S, Kanasaki K, Takeda-Watanabe A, Koya D. Sirtuins as possible drug targets in type 2 diabetes. Curr Drug Targets 2013;14:622-636. ArticlePubMed
- 16. Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab 2005;2:105-117. PubMed
- 17. Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell 2008;7:78-88. ArticlePubMed
- 18. Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 2007;6:307-319. ArticlePubMed
- 19. Zhang J. The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J Biol Chem 2007;282:34356-34364. ArticlePubMed
- 20. Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, Li X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol 2010;30:4712-4721. ArticlePubMedPMCPDF
- 21. Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol 2009;29:1363-1374. ArticlePubMedPDF
- 22. Yoshizaki T, Schenk S, Imamura T, Babendure JL, Sonoda N, Bae EJ, Oh DY, Lu M, Milne JC, Westphal C, Bandyopadhyay G, Olefsky JM. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am J Physiol Endocrinol Metab 2010;298:E419-E428. ArticlePubMed
- 23. Takeda-Watanabe A, Kitada M, Kanasaki K, Koya D. SIRT1 inactivation induces inflammation through the dysregulation of autophagy in human THP-1 cells. Biochem Biophys Res Commun 2012;427:191-196. ArticlePubMed
- 24. Xu F, Burk D, Gao Z, Yin J, Zhang X, Weng J, Ye J. Angiogenic deficiency and adipose tissue dysfunction are associated with macrophage malfunction in SIRT1-/- mice. Endocrinology 2012;153:1706-1716. ArticlePubMedPMCPDF
- 25. Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, Accili D. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 2012;150:620-632. ArticlePubMedPMC
- 26. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001;7:941-946. ArticlePubMedPDF
- 27. Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 2002;8:731-737. ArticlePubMedPDF
- 28. Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem 2006;281:39915-39924. PubMed
- 29. Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010;464:1313-1319. ArticlePubMedPDF
- 30. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004;350:664-671. ArticlePubMedPMC
- 31. Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 2007;13:332-339. ArticlePubMedPDF
- 32. Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci U S A 2001;98:3820-3825. ArticlePubMedPMC
- 33. Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 2005;437:1109-1111. ArticlePubMedPDF
- 34. Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J 3rd, Olefsky J, Guarente L, Montminy M. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 2008;456:269-273. ArticlePubMedPMCPDF
- 35. Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 2008;8:333-341. ArticlePubMedPMC
- 36. Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest 2011;121:4477-4490. ArticlePubMedPMC
- 37. Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev 2008;22:1753-1757. ArticlePubMedPMC
- 38. Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A 2007;104:12861-12866. ArticlePubMedPMC
- 39. Erion DM, Yonemitsu S, Nie Y, Nagai Y, Gillum MP, Hsiao JJ, Iwasaki T, Stark R, Weismann D, Yu XX, Murray SF, Bhanot S, Monia BP, Horvath TL, Gao Q, Samuel VT, Shulman GI. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc Natl Acad Sci U S A 2009;106:11288-11293. ArticlePubMedPMC
- 40. Nie Y, Erion DM, Yuan Z, Dietrich M, Shulman GI, Horvath TL, Gao Q. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat Cell Biol 2009;11:492-500. ArticlePubMedPMCPDF
- 41. Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, Wu SY, Chiang CM, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem 2010;285:33959-33970. ArticlePubMedPMC
- 42. Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell 2007;28:91-106. ArticlePubMed
- 43. Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, Tsang S, Wu SY, Chiang CM, Veenstra TD. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab 2009;10:392-404. ArticlePubMedPMC
- 44. Xu F, Gao Z, Zhang J, Rivera CA, Yin J, Weng J, Ye J. Lack of SIRT1 (Mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/- mice: a role of lipid mobilization and inflammation. Endocrinology 2010;151:2504-2514. ArticlePubMedPMCPDF
- 45. Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A 2008;105:9793-9798. ArticlePubMedPMC
- 46. Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 2011;14:612-622. ArticlePubMed
- 47. St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006;127:397-408. ArticlePubMed
- 48. Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Washida N, Tokuyama H, Hayashi K, Itoh H. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem Biophys Res Commun 2008;372:51-56. ArticlePubMed
- 49. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005;308:1043-1045. ArticlePubMedPMC
- 50. Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 2007;6:414-421. ArticlePubMed
- 51. Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 2008;134:329-340. ArticlePubMedPMC
- 52. Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008;134:317-328. ArticlePubMed
- 53. Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 2007;450:1086-1090. ArticlePubMedPDF
- 54. Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007;450:712-716. ArticlePubMedPMCPDF
- 55. Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, Mikolas E, Szijarto IA, Merei A, Halmai R, Meszaros LG, Sumegi B, Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr 2011;106:383-389. ArticlePubMed
- 56. Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW, Klein S. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab 2012;16:658-664. ArticlePubMedPMC
- 57. Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, Moller N, Jessen N, Pedersen SB, Jorgensen JO. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013;62:1186-1195. PubMedPMC
- 58. Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 2013;339:1216-1219. ArticlePubMedPMC
- 59. Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012;148:421-433. ArticlePubMedPMC
- 60. Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 2012;15:675-690. ArticlePubMedPMC
REFERENCES
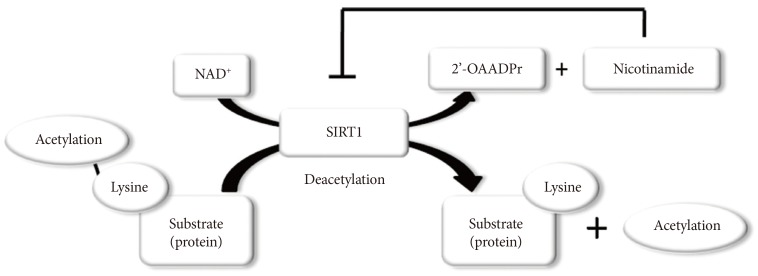
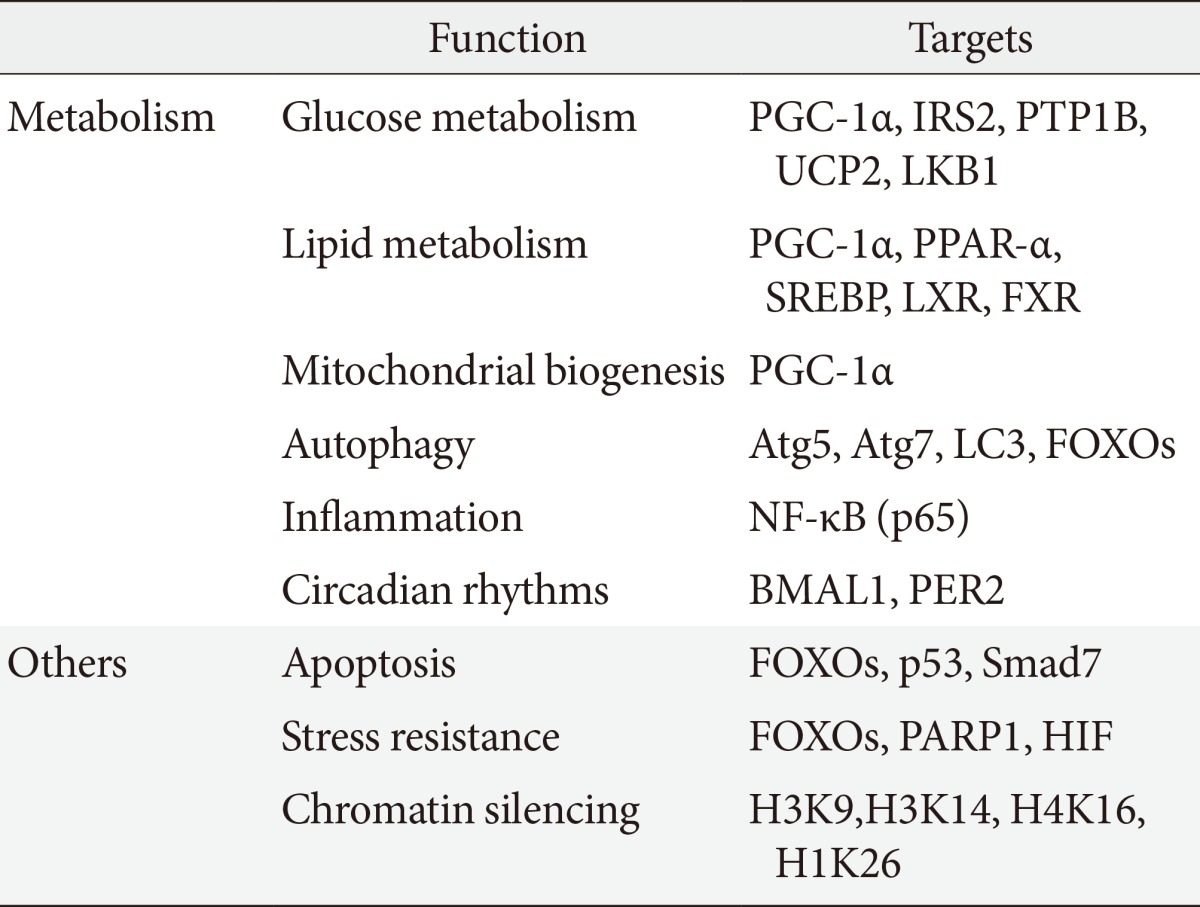
Sirtuin 1 (SIRT1) participates in the regulation of metabolism, including glucose/lipid metabolism, mitochondrial biogenesis, autophagy, inflammation, and circadian rhythms as well as other cellular functions, such as stress responses and apoptosis. SIRT1 also promote chromatin silencing. Many target proteins, such as transcription factors, transcriptional coregulatory proteins and several histones serve as the substrates for SIRT1.
PGC, peroxisome proliferator activated receptor-γ coactivator; IRS, insulin receptor substrate; PTP1B, protein tyrosine phosphatase 1B; UCP, uncoupling protein; LKB, liver kinase B; PPAR, peroxisome proliferator activated receptor; SREBP, sterol regulatory element binding protein; LXR, liver X receptor; FXR, farnesoid X receptor; Atg, autophagy-related gene; LC3, light chain 3; FOXO, forkhead box O; NF-κB, nuclear factor-κB; BMAL, brain and muscle aryl hydrocarbon receptor nuclear translocator-like; PER2, period 2; PARP, poly-ADP-ribose polymerase; HIF, hypoxia inducible factor.
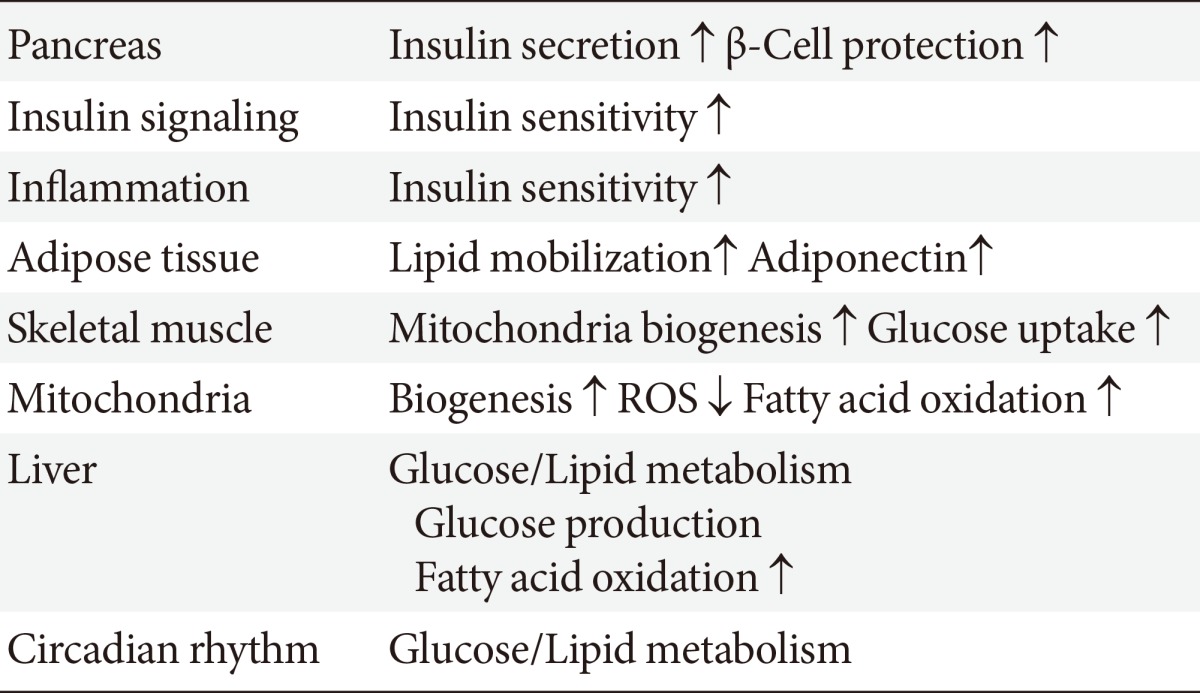
The proposed roles for sirtuin 1 (SIRT1) include regulating insulin secretion and β-cell protection, repression of the inflammation, and regulation of insulin signaling, mitochondrial biogenesis and subsequent reactive oxygen species (ROS) generation, adipogenesis, adiponectin secretion, hepatic glucose/lipid metabolism, and circadian rhythms. SIRT1 can improve insulin resistance and diabetic status.
Figure & Data
References
Citations

- SIRT1: a promising therapeutic target in type 2 diabetes mellitus
Ainaz Mihanfar, Maryam Akbarzadeh, Saber Ghazizadeh Darband, Shirin Sadighparvar, Maryam Majidinia
Archives of Physiology and Biochemistry.2024; 130(1): 13. CrossRef - The Role of Medicinal Plants in the Treatment and Management of Type
2 Diabetes
Tirna Paul, Kalyani Pathak, Riya Saikia, Urvashee Gogoi, Jon Jyoti Sahariah, Aparoop Das
Current Traditional Medicine.2024;[Epub] CrossRef - The favorable impacts of cardamom on related complications of diabetes: A comprehensive literature systematic review
Ramin Nasimi Doost Azgomi, Arash Karimi, Arezoo Moini Jazani
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2024; 18(2): 102947. CrossRef - Heat shock response during the resolution of inflammation and its progressive suppression in chronic-degenerative inflammatory diseases
Helena Trevisan Schroeder, Carlos Henrique De Lemos Muller, Thiago Gomes Heck, Mauricio Krause, Paulo Ivo Homem de Bittencourt
Cell Stress and Chaperones.2024; 29(1): 116. CrossRef - circGlis3 promotes β-cell dysfunction by binding to heterogeneous nuclear ribonucleoprotein F and encoding Glis3-348aa protein
Li Xiong, Yingying Gong, Huashan Liu, Liang Huang, Ziwei Zeng, Xiaobin Zheng, Wenxin Li, Zhenxing Liang, Liang Kang
iScience.2024; 27(1): 108680. CrossRef - Nephro‐protective effects of alpha‐lipoic acid in type I diabetes
Kriti Kushwaha, Debojyoti Mandal, Navneet Khurana, Jeena Gupta
Journal of Biochemical and Molecular Toxicology.2024;[Epub] CrossRef - Potential roles of ellagic acid on metabolic variables in diabetes mellitus: A systematic review
Vahid Maleki, Sepideh Abbaszadeh, Seyyed Morteza Seyyed Shoura, Asma Sohrabnavi, Mojtaba Sepandi, Maryam Taghdir
Clinical and Experimental Pharmacology and Physiology.2023; 50(2): 121. CrossRef - Agomelatine improves streptozotocin-induced diabetic nephropathy through melatonin receptors/SIRT1 signaling pathway
Nevertyty M. Mahmoud, Shimaa M. Elshazly, Arwa A. Hassan, Eman Soliman
International Immunopharmacology.2023; 115: 109646. CrossRef - Effect of Oleanolic acid administration on hepatic AMPK, SIRT-1, IL-6 and NF-κB levels in experimental diabetes
Hatice Iskender, Eda Dokumacioglu, Kubra Asena Terim Kapakin, Ismail Bolat, Behzat Mokhtare, Armagan Hayirli, Guler Yenice
Journal of Diabetes & Metabolic Disorders.2023; 22(1): 581. CrossRef - Antidiabetic activity of Berberis brandisiana is possibly mediated through modulation of insulin signaling pathway, inflammatory cytokines and adipocytokines in high fat diet and streptozotocin-administered rats
Shumaila Mehdi, Malik Hassan Mehmood, Mobeen Ghulam Ahmed, Usman Ali Ashfaq
Frontiers in Pharmacology.2023;[Epub] CrossRef - The Pathogenesis of Diabetes
Huiqin Guo, Haili Wu, Zhuoyu Li
International Journal of Molecular Sciences.2023; 24(8): 6978. CrossRef - Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms
Rabia Naz, Fatima Saqib, Samir Awadallah, Muqeet Wahid, Muhammad Farhaj Latif, Iram Iqbal, Mohammad S. Mubarak
Molecules.2023; 28(10): 3996. CrossRef - Pancreatic β-cell heterogeneity in adult human islets and stem cell-derived islets
Noura Aldous, Abu Saleh Md Moin, Essam M. Abdelalim
Cellular and Molecular Life Sciences.2023;[Epub] CrossRef - Mechanistic and Etiological Similarities in Diabetes Mellitus and
Alzheimer’s Disease: Antidiabetic Drugs as Optimistic Therapeutics in
Alzheimer’s Disease
Subham Das, Anu Kunnath Ramachandran, Debojyoti Halder, Saleem Akbar, Bahar Ahmed, Alex Joseph
CNS & Neurological Disorders - Drug Targets.2023; 22(7): 973. CrossRef - Resveratrol ameliorates triglyceride accumulation through FXR deacetylation in high glucose-treated HepG2 cells
Hao Yang, Yongjin Sun, Jinling Zhang, Shan Xu, Lidan Tang, Jinhong Gong, Hufeng Fang, Ying Lin, Jie Ren, Dan Su
Journal of Functional Foods.2023; 107: 105679. CrossRef - A Potential Role for Sirtuin-1 in Alzheimer’s Disease: Reviewing the Biological and Environmental Evidence
Mehrane Mehramiz, Tenielle Porter, Eleanor K. O’Brien, Stephanie R. Rainey-Smith, Simon M. Laws, Craig Atwood
Journal of Alzheimer's Disease Reports.2023; 7(1): 823. CrossRef - Pathophysiology of diabetic kidney disease and autophagy: A review
Jiawei Yu, Yan Liu, Hongjie Li, Peirong Zhang
Medicine.2023; 102(30): e33965. CrossRef - Low sirtuin-1 levels are associated with gestational diabetes mellitus
Hasan ULUBASOGLU, Necati HANCERLIOGULLARI, Aytekin TOKMAK, Levent H. KESKIN, Tuba CANDAR, Ozlem MORALOGLU TEKIN
Minerva Endocrinology.2023;[Epub] CrossRef - Effect of resistance training and high-intensity interval training on metabolic parameters and serum level of Sirtuin1 in postmenopausal women with metabolic syndrome: a randomized controlled trial
Saeid Shamlou Kazemi, Ali Heidarianpour, Elnaz Shokri
Lipids in Health and Disease.2023;[Epub] CrossRef - Purple Napiergrass (Pennisetum purpureum Schumach) Hot Water Extracts Ameliorate High-Fat Diet-Induced Obesity and Metabolic Disorders in Mice
Pin-Yu Ho, Yen-Chun Koh, Ting-Jang Lu, Po-Lin Liao, Min-Hsiung Pan
Journal of Agricultural and Food Chemistry.2023; 71(51): 20701. CrossRef - The role of sirtuins in the regulatin of oxidative stress during the progress and therapy of type 2 diabetes mellitus
Jiawen Chen, Qi Wang, Ruiyan Li, Zhe Li, Qizhou Jiang, Fangrong Yan, Junmei Ye
Life Sciences.2023; 333: 122187. CrossRef - Investigation of polymorphism role in protein structure and function for selected cancer and diabetes disease; a rationale to selection of targets for insilico drug screening
Christopher Busayo Olowosoke, Tope Abraham Ibisanmi, Chioma Joy Eze, Abayomi Abiodun Shofunde, Tomiwa Lois Olubena, Olalekan Akadiri
Informatics in Medicine Unlocked.2023; 42: 101342. CrossRef - Folic Acid and Folinic Acid Protect Hearts of Aging Triple-transgenic Alzheimer’s Disease mice via IGF1R/PI3K/AKT and SIRT1/AMPK Pathways
Da-Tong Ju, Rwei-Fen S. Huang, Bruce Chi-Kang Tsai, Yi-Chen Su, Ping-Ling Chiu, Yung-Ming Chang, V. Vijaya Padma, Tsung-Jung Ho, Chun-Hsu Yao, Wei-Wen Kuo, Chih-Yang Huang
Neurotoxicity Research.2023; 41(6): 648. CrossRef - Role of microRNA-34a in blood–brain barrier permeability and mitochondrial function in ischemic stroke
Cole T. Payne, Sidra Tabassum, Silin Wu, Heng Hu, Aaron M. Gusdon, Huimahn A. Choi, Xuefang S. Ren
Frontiers in Cellular Neuroscience.2023;[Epub] CrossRef - Resveratrol and Markers of Polycystic Ovary Syndrome: a Systematic Review of Animal and Clinical Studies
Sara Shojaei-Zarghani, Maryam Rafraf
Reproductive Sciences.2022; 29(9): 2477. CrossRef - Efficacy of artemisinin and its derivatives in animal models of type 2 diabetes mellitus: A systematic review and meta-analysis
Tingchao Wu, Haoyue Feng, Mingmin He, Rensong Yue, Shaoqi Wu
Pharmacological Research.2022; 175: 105994. CrossRef - The effect of 12 weeks of training in water on serum levels of SIRT1 and FGF-21, glycemic index, and lipid profile in patients with type 2 diabetes
Bahram Jamali Gharakhanlou, Solmaz Babaei Bonab
International Journal of Diabetes in Developing Countries.2022; 42(4): 727. CrossRef - New insights into the role of melatonin in diabetic cardiomyopathy
Keming Huang, Xianling Luo, Yi Zhong, Li Deng, Jian Feng
Pharmacology Research & Perspectives.2022;[Epub] CrossRef - Obesity and Male Reproduction: Do Sirtuins Play a Role?
Federica Barbagallo, Sandro La Vignera, Rossella Cannarella, Laura M. Mongioì, Vincenzo Garofalo, Claudia Leanza, Marta Marino, Aldo E. Calogero, Rosita A. Condorelli
International Journal of Molecular Sciences.2022; 23(2): 973. CrossRef - Nicotinamide mononucleotide promotes pancreatic islet function through the SIRT1 pathway in mice after severe burns
Xinzhu Liu, Dawei Li, Zhaoxing Liu, Yaoyao Song, Bohan Zhang, Yu Zang, Wen Zhang, Yuezeng Niu, Chuan’an Shen
Burns.2022; 48(8): 1922. CrossRef - d-Allulose Ameliorates Hyperglycemia Through IRE1α Sulfonation-RIDD-Sirt1 Decay Axis in the Skeletal Muscle
Hwa-Young Lee, Geum-Hwa Lee, The-Hiep Hoang, Seon-Ah Park, Juwon Lee, Junghyun Lim, Soonok Sa, Go Eun Kim, Jung Sook Han, Junghyun Kim, Han-Jung Chae
Antioxidants & Redox Signaling.2022; 37(4-6): 229. CrossRef - GABA and Fermented Curcuma longa L. Extract Enriched with GABA Ameliorate Obesity through Nox4-IRE1α Sulfonation-RIDD-SIRT1 Decay Axis in High-Fat Diet-Induced Obese Mice
Hwa-Young Lee, Geum-Hwa Lee, The-Hiep Hoang, Yu-Mi Kim, Gi-Hyun Jang, Chang-Hwan Seok, Yun-Geum-Sang Gwak, Junghyun Lim, Junghyun Kim, Han-Jung Chae
Nutrients.2022; 14(8): 1680. CrossRef - Resveratrol regulates Hsp60 in HEK 293T cells during activation of SIRT1 revealed by nascent protein labeling strategy
Tian Su, Zhen Wang, Zhengyi Zhang, Zhanwu Hou, Xiao Han, Fei Yang, Huadong Liu
Food & Nutrition Research.2022;[Epub] CrossRef - Effect of cardamom supplementation on a number of metabolic factors: A systematic review and meta-analysis
Ghazaleh Nameni, Yousef Moradi, Marsa Zaroudi, Sanaz Jamshidi
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2022; 16(6): 102523. CrossRef - The mitochondrial unfolded protein response: A multitasking giant in the fight against human diseases
Zixin Zhou, Yumei Fan, Ruikai Zong, Ke Tan
Ageing Research Reviews.2022; 81: 101702. CrossRef - Cardioprotective Effects of 1-(3,6-Dibromo-carbazol-9-yl)-3-Phenylamino-Propan-2-Ol in Diabetic Hearts via Nicotinamide Phosphoribosyltransferase Activation
Jared Tur, Sachin L. Badole, Ravikumar Manickam, Kalyan C. Chapalamadugu, Wanling Xuan, Wayne Guida, Jaret J. Crews, Kirpal S. Bisht, Srinivas M. Tipparaju
Journal of Pharmacology and Experimental Therapeutics.2022; 382(2): 233. CrossRef - Celastrol: A Promising Agent Fighting against Cardiovascular Diseases
Zhexi Li, Jingyi Zhang, Xulei Duan, Guoan Zhao, Min Zhang
Antioxidants.2022; 11(8): 1597. CrossRef - The role of Sirtuin 1 in the pathophysiology of polycystic ovary syndrome
Mali Wu, Jie Zhang, Ran Gu, Fangfang Dai, Dongyong Yang, Yajing Zheng, Wei Tan, Yifan Jia, Bingshu Li, Yanxiang Cheng
European Journal of Medical Research.2022;[Epub] CrossRef - Anti-apoptotic properties of N-Acetyl cysteine and its effects on of Liver X receptor and Sirtuin 1 expression in the liver of rats exposed to Lead
Asghar Beigi Harchegani, Sareh Rostami, Zhaleh Mohsenifar, Alireza Beheshti Dafchahi, Fatemeh Mozaffari Moghadam, Mohammad Jaafarzadeh, Saman Seyfizadeh Saraabestan, Najmeh Ranji
Journal of Trace Elements in Medicine and Biology.2022; 74: 127070. CrossRef - Caloric Restriction, Friend or Foe: Effects on Metabolic Status in Association with the Intestinal Microbiome and Metabolome
Xiangnan Zhang, Lin Shi, Qiannan Li, Chaofan Song, Ning Han, Tao Yan, Liansheng Zhang, Daoyuan Ren, Yan Zhao, Xingbin Yang
Journal of Agricultural and Food Chemistry.2022; 70(43): 14061. CrossRef - Effects of Fruit and Vegetable Polyphenols on the Glycemic Control and Metabolic Parameters in Type 2 Diabetes Mellitus: A Review
Che Anis Jauharah Che Mohd Zin, Wan Mohd Izani Wan Mohamed, Nurzalina Abdul Karim Khan, Wan Rosli Wan Ishak
Preventive Nutrition and Food Science.2022; 27(3): 257. CrossRef - Berberine mitigates hepatic insulin resistance by enhancing mitochondrial architecture via the SIRT1/Opa1 signalling pathway
Jia Xu, Yining Zhang, Zhiyi Yu, Yueqi Guan, Yuqian Lv, Meishuang Zhang, Ming Zhang, Li Chen, Xiaoyan Lv, Fengying Guan
Acta Biochimica et Biophysica Sinica.2022; 54(10): 1464. CrossRef - The Role of p66Shc in Diabetes: A Comprehensive Review from Bench to Bedside
SeyedehFatemeh Mousavi, Mohammad Amin Khazeei Tabari, Alireza Bagheri, Noosha Samieefar, Negar Shaterian, Roya Kelishadi, Eusebio Chiefari
Journal of Diabetes Research.2022; 2022: 1. CrossRef - Systematic Insight of Resveratrol Activated SIRT1 Interactome through Proximity Labeling Strategy
Tian Su, Zhengyi Zhang, Xiao Han, Fei Yang, Zhen Wang, Ying Cheng, Huadong Liu
Antioxidants.2022; 11(12): 2330. CrossRef - Effects of Resveratrol Supplementation and Exercise on Apoptosis, Lipid Profile, and Expression of Farnesoid X Receptor, Liver X Receptor and Sirtuin 1 Genes in the Liver of Type 1 Diabetic Rats
Ali Nouri, Parvin Farzanegi, Mohammad Ali Azarbayjani
Medical Laboratory Journal.2022; 16(4): 39. CrossRef - SIRT1 functional polymorphisms (rs12778366, rs3758391) as genetic biomarkers of susceptibility to type 2 diabetes mellitus in Iranians: a case-control study and computational analysis
Mohammad Bagher Sadeghi, Alireza Nakhaee, Ramin Saravani, Mohammad Hassan Sadeghi, Saman Sargazi, Milad Heidari Nia
International Journal of Diabetes in Developing Countries.2021; 41(3): 447. CrossRef - Elucidation of Structural Determinants Delineates the Residues Playing Key Roles in Differential Dynamics and Selective Inhibition of Sirt1–3
Mrityunjay Singh, Mitul Srivastava, Sharad R. Wakode, Shailendra Asthana
Journal of Chemical Information and Modeling.2021; 61(3): 1105. CrossRef - Discovery and characterization of small molecule SIRT3-specific inhibitors as revealed by mass spectrometry
Saurabh Loharch, Sonali Chhabra, Abhinit Kumar, Sapna Swarup, Raman Parkesh
Bioorganic Chemistry.2021; 110: 104768. CrossRef - Exploring the recent molecular targets for diabetes and associated complications
Amit Gupta, Tapan Behl, Aayush Sehgal, Shaveta Bhardwaj, Sukhbir Singh, Neelam Sharma, Abdul Hafeez
Molecular Biology Reports.2021; 48(3): 2863. CrossRef - Opportunities and challenges of algal fucoidan for diabetes management
Yuxi Wen, Luying Gao, Hengsheng Zhou, Chao Ai, Xiaozhou Huang, Mingfu Wang, Yuyu Zhang, Chao Zhao
Trends in Food Science & Technology.2021; 111: 628. CrossRef - The effect of N-Acetyl cysteine on the expression of Fxr (Nr1h4), LXRα (Nr1h3) and Sirt1 genes, oxidative stress, and apoptosis in the liver of rats exposed to different doses of cadmium
Zahra Azarmehr, Najmeh Ranji, Zeinab Khazaei Koohpar, Hadi Habibollahi
Molecular Biology Reports.2021; 48(3): 2533. CrossRef - Maternal obesity as a risk factor for developing diabetes in offspring: An epigenetic point of view
Simon Lecoutre, Salwan Maqdasy, Christophe Breton
World Journal of Diabetes.2021; 12(4): 366. CrossRef - Sirtuin 1 ve Sirtuin 2’nin Tip 2 Diyabet ile İlişkisi
İlke ULU, Güneş ÇAKMAK GENÇ, Sevim KARAKAŞ ÇELİK
Turkish Journal of Diabetes and Obesity.2021; 5(1): 81. CrossRef - Human SHC‐transforming protein 1 and its isoforms p66shc: A novel marker for prediabetes
Herbert F Jelinek, Charlotte Helf, Kinda Khalaf
Journal of Diabetes Investigation.2021; 12(10): 1881. CrossRef - An Insight into Giant Cell Arteritis Pathogenesis: Evidence for Oxidative Stress and SIRT1 Downregulation
Alessandro Ianni, Poonam Kumari, Shahriar Tarighi, Flavia Rita Argento, Eleonora Fini, Giacomo Emmi, Alessandra Bettiol, Thomas Braun, Domenico Prisco, Claudia Fiorillo, Matteo Becatti
Antioxidants.2021; 10(6): 885. CrossRef - Case Report of Impaired Fasting Glucose Improved with Korean Medicine Treatment and Dietetic Therapy
Eun-mi Kim, Ki-tae Kim
The Journal of Internal Korean Medicine.2021; 42(2): 175. CrossRef - Can resveratrol modulate sirtuins in obesity and related diseases? A systematic review of randomized controlled trials
Gabriela Macedo Fraiz, Aline Rosignoli da Conceição, Darlene Larissa de Souza Vilela, Daniela Mayumi Usuda Prado Rocha, Josefina Bressan, Helen Hermana Miranda Hermsdorff
European Journal of Nutrition.2021; 60(6): 2961. CrossRef - Ameliorative effects of fisetin in letrozole-induced rat model of polycystic ovary syndrome
Aynaz Mihanfar, Mohammad Nouri, Leila Roshangar, Mohammad Hassan Khadem-Ansari
The Journal of Steroid Biochemistry and Molecular Biology.2021; 213: 105954. CrossRef - The effect of daily intake of vitamin D-fortified yogurt drink, with and without added calcium, on serum adiponectin and sirtuins 1 and 6 in adult subjects with type 2 diabetes
Bahareh Nikooyeh, Bruce W. Hollis, Tirang R. Neyestani
Nutrition & Diabetes.2021;[Epub] CrossRef - Up-regulation of GLP-1R improved the dysfunction of late EPCs under hyperglycemia by regulating SIRT1 expression
Qiang Tu, Jun-feng Wang, Hua-qiang Xie, Qi Zhao, Jie Fu, Hua-lin Xu, Zheng Cao
Molecular and Cellular Endocrinology.2021; 538: 111455. CrossRef - Therapeutic Screening of Herbal Remedies for the Management of Diabetes
Mahmoud Balbaa, Marwa El-Zeftawy, Shaymaa A. Abdulmalek
Molecules.2021; 26(22): 6836. CrossRef - microRNA‐141 is associated with hepatic steatosis by downregulating the sirtuin1/AMP‐activated protein kinase pathway in hepatocytes
Zeynab Yousefi, Mitra Nourbakhsh, Zohreh Abdolvahabi, Seyedeh‐Sara Ghorbanhosseini, Zahra Hesari, Sahar Yarahmadi, Samira Ezzati‐Mobasser, Parvane Seiri, Mohammad Borji, Reza Meshkani, Mojtaba Malek
Journal of Cellular Physiology.2020; 235(2): 880. CrossRef - Trifolium pratense (Red Clover) Improve SIRT1 Expression and Glycogen Content in High Fat Diet‐Streptozotocin Induced Type 2 Diabetes in Rats
Manisha J. Oza, Yogesh A. Kulkarni
Chemistry & Biodiversity.2020;[Epub] CrossRef - Additive Effect of Resveratrol on Astrocyte Swelling Post-exposure to Ammonia, Ischemia and Trauma In Vitro
Mehran Taherian, Michael D. Norenberg, Kiran S. Panickar, Nagarajarao Shamaladevi, Anis Ahmad, Purbasha Rahman, Arumugam R. Jayakumar
Neurochemical Research.2020; 45(5): 1156. CrossRef - Association of the rs3758391 polymorphism in the SIRT1 gene with diabetic nephropathy and decreased estimated glomerular filtration rate (GFR) in a population from southwest Iran
Ramin Tavakoli Faradonbeh, Mehrnoosh Zakerkish, Ali Karimi Akhormeh, Narges Mohammadtaghvaei, Mohammad Taha Jalali, Hamid Yaghooti
International Journal of Diabetes in Developing Countries.2020; 40(1): 99. CrossRef - Hydrogen Sulfide Ameliorates Lung Ischemia-Reperfusion Injury Through SIRT1 Signaling Pathway in Type 2 Diabetic Rats
Tao Jiang, Weiwei Yang, Hongli Zhang, Zhiqiang Song, Tianhua Liu, Xiangqi Lv
Frontiers in Physiology.2020;[Epub] CrossRef - Lycium barbarum Polysaccharides Improve Testicular Spermatogenic Function in Streptozotocin-Induced Diabetic Rats
Xiaocan Lei, Peng Huo, Yaohui Wang, Yuanjie Xie, Qingxiang Shi, Haoyan Tu, Jun Yao, Zhongcheng Mo, Shun Zhang
Frontiers in Endocrinology.2020;[Epub] CrossRef - Maya gene variants related to the risk of type 2 diabetes in a family-based association study
Miriam G. Domínguez-Cruz, María de Lourdes Muñoz, Armando Totomoch-Serra, María G. García-Escalante, Juan Burgueño, Nina Valadez-González, Doris Pinto-Escalante, Alvaro Díaz-Badillo
Gene.2020; 730: 144259. CrossRef - Development of activity-based probes for the protein deacylase Sirt1
Christopher J. Goetz, Daniel J. Sprague, Brian C. Smith
Bioorganic Chemistry.2020; 104: 104232. CrossRef - Celastrol attenuates inflammatory responses in adipose tissues and improves skeletal muscle mitochondrial functions in high fat diet-induced obese rats via upregulation of AMPK/SIRT1 signaling pathways
Mohamad Hafizi Abu Bakar, Khairul Anuar Shariff, Joo Shun Tan, Lai Kuan Lee
European Journal of Pharmacology.2020; 883: 173371. CrossRef - SCD1 regulates the AMPK/SIRT1 pathway and histone acetylation through changes in adenine nucleotide metabolism in skeletal muscle
Anna Dziewulska, Aneta M. Dobosz, Agnieszka Dobrzyn, Agnieszka Smolinska, Katarzyna Kolczynska, James M. Ntambi, Pawel Dobrzyn
Journal of Cellular Physiology.2020; 235(2): 1129. CrossRef - Effect of Quamoclit angulata Extract Supplementation on Oxidative Stress and Inflammation on Hyperglycemia-Induced Renal Damage in Type 2 Diabetic Mice
Ji Eun Park, Heaji Lee, Hyunkyung Rho, Seong Min Hong, Sun Yeou Kim, Yunsook Lim
Antioxidants.2020; 9(6): 459. CrossRef - Effect of green cardamom on lipoproteins, glycemic control and anthropometric parameters: A meta-analysis of randomized clinical trials
Omid Asbaghi, Elham Eslampour, Željko Reiner, Bita Badehnoosh, Fariba Kolahdooz, Sajjad Moradi, Shahrzad Hashemi Dizaji, Zatollah Asemi
Clinical Nutrition ESPEN.2020; 37: 24. CrossRef - The Potential Roles of Artemisinin and Its Derivatives in the Treatment of Type 2 Diabetes Mellitus
Ya-yi Jiang, Jia-cheng Shui, Bo-xun Zhang, Jia-wei Chin, Ren-song Yue
Frontiers in Pharmacology.2020;[Epub] CrossRef - Adipose Tissue SIRT1 Regulates Insulin Sensitizing and Anti-Inflammatory Effects of Berberine
Yun Shan, Shuchen Zhang, Bin Gao, Shu Liang, Hao Zhang, Xizhong Yu, Juan Zhao, Lifang Ye, Qin Yang, Wenbin Shang
Frontiers in Pharmacology.2020;[Epub] CrossRef - Inhibition of the SIRT1 signaling pathway exacerbates endoplasmic reticulum stress induced by renal ischemia/reperfusion injury in type 1 diabetic rats
Jianjian Zhang, Lei Wang, Daojing Gong, Yuanyuan Yang, Xiuheng Liu, Zhiyuan Chen
Molecular Medicine Reports.2019;[Epub] CrossRef - Beneficial effects of green cardamom on serum SIRT1, glycemic indices and triglyceride levels in patients with type 2 diabetes mellitus: a randomized double‐blind placebo controlled clinical trial
Mohadeseh Aghasi, Fariba Koohdani, Mostafa Qorbani, Ensieh Nasli‐Esfahani, Shohreh Ghazi‐Zahedi, Hoorieh Khoshamal, Ali Keshavarz, Gity Sotoudeh
Journal of the Science of Food and Agriculture.2019; 99(8): 3933. CrossRef - A Review of Type 2 Diabetes Mellitus Predisposing Genes
Tajudeen O. Yahaya, Titilola F. Salisu
Current Diabetes Reviews.2019; 16(1): 52. CrossRef - Exercise training upregulates SIRT1 to attenuate inflammation and metabolic dysfunction in kidney and liver of diabetic db/db mice
Hung-Wen Liu, Hao-Han Kao, Chi-Hang Wu
Nutrition & Metabolism.2019;[Epub] CrossRef - Resveratrol: from enhanced biosynthesis and bioavailability to multitargeting chronic diseases
Naveet Pannu, Archana Bhatnagar
Biomedicine & Pharmacotherapy.2019; 109: 2237. CrossRef - The Effect of Resveratrol Supplementation on Cardio‐Metabolic Risk Factors in Patients with Type 2 Diabetes: A Randomized, Double‐Blind Controlled Trial
Shima Abdollahi, Amin Salehi‐Abargouei, Omid Toupchian, Mohammad Hasan Sheikhha, Hossein Fallahzadeh, Masoud Rahmanian, Mahtab Tabatabaie, Hassan Mozaffari‐Khosravi
Phytotherapy Research.2019; 33(12): 3153. CrossRef - Sirtuins and diabetes: optimizing the sweetness in the blood
Abhinav Kanwal, Liston Augustine Dsouza
Translational Medicine Communications.2019;[Epub] CrossRef - In silico and in vitro identification of candidate SIRT1 activators from Indonesian medicinal plants compounds database
Azminah Azminah, Linda Erlina, Maksum Radji, Abdul Mun’im, Rezi Riadhi Syahdi, Arry Yanuar
Computational Biology and Chemistry.2019; 83: 107096. CrossRef - Ginsenoside Rb1 Attenuates High Glucose-Induced Oxidative Injury via the NAD-PARP-SIRT Axis in Rat Retinal Capillary Endothelial Cells
Chunlan Fan, Qing Ma, Meng Xu, Yuan Qiao, Yi Zhang, Pin Li, Yucong Bi, Minke Tang
International Journal of Molecular Sciences.2019; 20(19): 4936. CrossRef - Effects and Underlying Mechanisms of Bioactive Compounds on Type 2 Diabetes Mellitus and Alzheimer’s Disease
Rongzi Li, Yuxian Zhang, Suhail Rasool, Thangiah Geetha, Jeganathan Ramesh Babu
Oxidative Medicine and Cellular Longevity.2019; 2019: 1. CrossRef - Selection of reference genes for miRNA quantitative PCR and its application in miR-34a/Sirtuin-1 mediated energy metabolism in Megalobrama amblycephala
Jie Liu, Erteng Jia, Huajuan Shi, Xiangfei Li, Guangzhen Jiang, Cheng Chi, Wenbin Liu, Dingdong Zhang
Fish Physiology and Biochemistry.2019; 45(5): 1663. CrossRef - Fucoxanthin Alleviates Oxidative Stress through Akt/Sirt1/FoxO3α Signaling to Inhibit HG-Induced Renal Fibrosis in GMCs
Guanyu Yang, Lin Jin, Dongxiao Zheng, Xiaoliang Tang, Junwei Yang, Lingxuan Fan, Xi Xie
Marine Drugs.2019; 17(12): 702. CrossRef - Melatonin attenuates acute kidney ischemia/reperfusion injury in diabetic rats by activation of the SIRT1/Nrf2/HO-1 signaling pathway
Si Shi, Shaoqing Lei, Chaoliang Tang, Kai Wang, Zhongyuan Xia
Bioscience Reports.2019;[Epub] CrossRef - Rhizoma Paridis total saponins alleviate H2O2‑induced oxidative stress injury by upregulating the Nrf2 pathway
Baocheng Zhao, Zhenjun Wang, Jiagang Han, Guanghui Wei, Bingqiang Yi, Zhulin Li
Molecular Medicine Reports.2019;[Epub] CrossRef - Role of sirtuin-1 in diabetic nephropathy
Wanning Wang, Weixia Sun, Yanli Cheng, Zhonggao Xu, Lu Cai
Journal of Molecular Medicine.2019; 97(3): 291. CrossRef - Sirtuins and Type 2 Diabetes: Role in Inflammation, Oxidative Stress, and Mitochondrial Function
Munehiro Kitada, Yoshio Ogura, Itaru Monno, Daisuke Koya
Frontiers in Endocrinology.2019;[Epub] CrossRef - Mechanistic insights into the effect of lutein on atherosclerosis, vascular dysfunction, and related risk factors: A systematic review of in vivo, ex vivo and in vitro studies
Fatemeh Hajizadeh-Sharafabad, Zohreh Ghoreishi, Vahid Maleki, Ali Tarighat-Esfanjani
Pharmacological Research.2019; 149: 104477. CrossRef - Chronic whole-body heat treatment relieves atherosclerotic lesions, cardiovascular and metabolic abnormalities, and enhances survival time restoring the anti-inflammatory and anti-senescent heat shock response in mice
Maciel Alencar Bruxel, Angela Maria Vicente Tavares, Luiz Domingues Zavarize Neto, Victor de Souza Borges, Helena Trevisan Schroeder, Patricia Martins Bock, Maria Inês Lavina Rodrigues, Adriane Belló-Klein, Paulo Ivo Homem de Bittencourt
Biochimie.2019; 156: 33. CrossRef - Swimming training by affecting the pancreatic Sirtuin1 (SIRT1) and oxidative stress, improves insulin sensitivity in diabetic male rats
Rafighe Ghiasi, Roya Naderi, Roghayeh Sheervalilou, Mohammad Reza Alipour
Hormone Molecular Biology and Clinical Investigation.2019;[Epub] CrossRef - Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced “Stemness” through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration
Michalina Alicka, Piotr Major, Michał Wysocki, Krzysztof Marycz
Journal of Clinical Medicine.2019; 8(6): 765. CrossRef - A novel recombinant peptide INSR-IgG4Fc (Yiminsu) restores insulin sensitivity in experimental insulin resistance models
Jing Wang, Zhe Shi, Tao Zou, Min-Xu Zou, Hui-Xian Yang, Cai-Ping Zhang, De-Biao Xiang, Li-Mei Lin, Hui-Yu Liu, De-yu Fang, Duan-Fang Liao
Biomedicine & Pharmacotherapy.2019; 109: 1276. CrossRef - Immunometabolism and Pulmonary Infections: Implications for Protective Immune Responses and Host-Directed Therapies
Martin Rao, Ernest Dodoo, Alimuddin Zumla, Markus Maeurer
Frontiers in Microbiology.2019;[Epub] CrossRef - Salvianolic Acid B Attenuates Apoptosis of HUVEC Cells Treated with High Glucose or High Fat via Sirt1 Activation
Jinghui Zhai, Lina Tao, Yueming Zhang, Huan Gao, Xiaoyu Qu, Yanqing Song, Sixi Zhang
Evidence-Based Complementary and Alternative Medicine.2019; 2019: 1. CrossRef - Obesity programmed by prenatal dexamethasone and postnatal high-fat diet leads to distinct alterations in nutrition sensory signals and circadian-clock genes in visceral adipose tissue
Ching-Chou Tsai, Mao-Meng Tiao, Jiunn-Ming Sheen, Li-Tung Huang, You-Lin Tain, I-Chun Lin, Yu-Ju Lin, Yun-Ju Lai, Chih-Cheng Chen, Kow-Aung Chang, Hong-Ren Yu
Lipids in Health and Disease.2019;[Epub] CrossRef - Mapping Research in the Obesity, Adipose Tissue, and MicroRNA Field: A Bibliometric Analysis
João Manoel Alves, Ramon Handerson Gomes Teles, Camila do Valle Gomes Gatto, Vitor Rosetto Muñoz, Márcia Regina Cominetti, Ana Cláudia Garcia de Oliveira Duarte
Cells.2019; 8(12): 1581. CrossRef - Chronic hypoglycemic effect and phytochemical composition of Smilax moranensis roots
Adriana Romo-Pérez, Sonia Marlen Escandón-Rivera, Adolfo Andrade-Cetto
Revista Brasileira de Farmacognosia.2019; 29(2): 246. CrossRef - Prevention of kidney cell damage in hyperglycaemia condition by adiponectin
Sajad Esmaeili, Maryam Motamedrad, Mina Hemmati, Omid Mehrpour, Mohsen Khorashadizadeh
Cell Biochemistry and Function.2019; 37(3): 148. CrossRef - Molecular Mechanism of the Protective Effect of Zerumbone on Lipopolysaccharide-Induced Inflammation of THP-1 Cell-Derived Macrophages
Min-Ju Kim, Jung-Mi Yun
Journal of Medicinal Food.2019; 22(1): 62. CrossRef - Impairment of PPARα and the Fatty Acid Oxidation Pathway Aggravates Renal Fibrosis during Aging
Ki Wung Chung, Eun Kyeong Lee, Mi Kyung Lee, Goo Taeg Oh, Byung Pal Yu, Hae Young Chung
Journal of the American Society of Nephrology.2018; 29(4): 1223. CrossRef - Pomegranate Juice Increases Sirtuin1 Protein in Peripheral Blood Mononuclear Cell from Patients with Type 2 Diabetes: A Randomized Placebo Controlled Clinical Trial
Golbon Sohrab, Javad Nasrollahzadeh, Maryam Tohidi, Hamid Zand, Omid Nikpayam
Metabolic Syndrome and Related Disorders.2018; 16(8): 446. CrossRef - Effect of resveratrol treatment on graft revascularization after islet transplantation in streptozotocin-induced diabetic mice
Eun-Mi Lee, Inwon Park, Ye-Jee Lee, Young-Hye You, Ji-Won Kim, Myung-Jun Kim, Yu-Bae Ahn, Pilhan Kim, Seung-Hyun Ko
Islets.2018; 10(1): 25. CrossRef - miR‐221 negatively regulates inflammation and insulin sensitivity in white adipose tissue by repression of sirtuin‐1 (SIRT1)
Jie Peng, Yuanfei Zhou, Zhao Deng, Hong Zhang, Yinghui Wu, Tongxing Song, Yang Yang, Hongkui Wei, Jian Peng
Journal of Cellular Biochemistry.2018; 119(8): 6418. CrossRef - Molecular Imaging of Sirtuin1 Expression–Activity in Rat Brain Using Positron-Emission Tomography–Magnetic-Resonance Imaging with [18F]-2-Fluorobenzoylaminohexanoicanilide
Robin Bonomi, Vadim Popov, Maxwell T. Laws, David Gelovani, Anjoy Majhi, Aleksandr Shavrin, Xin Lu, Otto Muzik, Nashaat Turkman, Renshyan Liu, Thomas Mangner, Juri G. Gelovani
Journal of Medicinal Chemistry.2018; 61(16): 7116. CrossRef - Vitamin B12 supplementation influences methylation of genes associated with Type 2 diabetes and its intermediate traits
Dilip K Yadav, Smeeta Shrestha, Karen A Lillycrop, Charu V Joglekar, Hong Pan, Joanna D Holbrook, Caroline HD Fall, Chittaranjan S Yajnik, Giriraj R Chandak
Epigenomics.2018; 10(1): 71. CrossRef - The effects of green cardamom supplementation on blood glucose, lipids profile, oxidative stress, sirtuin-1 and irisin in type 2 diabetic patients: a study protocol for a randomized placebo-controlled clinical trial
Mohadeseh Aghasi, Shohreh Ghazi-Zahedi, Fariba Koohdani, Fereydoun Siassi, Ensieh Nasli-Esfahani, Ali Keshavarz, Mostafa Qorbani, Hoorieh Khoshamal, Asma Salari-Moghaddam, Gity Sotoudeh
BMC Complementary and Alternative Medicine.2018;[Epub] CrossRef - LincRNA 1700020I14Rik alleviates cell proliferation and fibrosis in diabetic nephropathy via miR-34a-5p/Sirt1/HIF-1α signaling
Ailing Li, Rui Peng, Yan Sun, Handeng Liu, Huimin Peng, Zheng Zhang
Cell Death & Disease.2018;[Epub] CrossRef - Treatment of NASH with Antioxidant Therapy: Beneficial Effect of Red Cabbage on Type 2 Diabetic Rats
Stéphanie Dal, Remmelt Van der Werf, Catherine Walter, William Bietiger, Elodie Seyfritz, Carole Mura, Claude Peronet, Julie Legrandois, Dalal Werner, Said Ennahar, Fabien Digel, Maillard-Pedracini Elisa, Michel Pinget, Nathalie Jeandidier, Eric Marchioni
Oxidative Medicine and Cellular Longevity.2018; 2018: 1. CrossRef - Formononetin Treatment in Type 2 Diabetic Rats Reduces Insulin Resistance and Hyperglycemia
Manisha J. Oza, Yogesh A. Kulkarni
Frontiers in Pharmacology.2018;[Epub] CrossRef - Tocotrienol-rich fraction supplementation reduces hyperglycemia-induced skeletal muscle damage through regulation of insulin signaling and oxidative stress in type 2 diabetic mice
Heaji Lee, Yunsook Lim
The Journal of Nutritional Biochemistry.2018; 57: 77. CrossRef - Circulating soluble RAGE isoforms are attenuated in obese, impaired-glucose-tolerant individuals and are associated with the development of type 2 diabetes
Edwin R. Miranda, Vikram S. Somal, Jacob T. Mey, Brian K. Blackburn, Edward Wang, Sarah Farabi, Kristian Karstoft, Ciaran E. Fealy, Sangeeta Kashyap, John P. Kirwan, Laurie Quinn, Thomas P. J. Solomon, Jacob M. Haus
American Journal of Physiology-Endocrinology and Metabolism.2017; 313(6): E631. CrossRef - Combination Treatments with Luteolin and Fisetin Enhance Anti-Inflammatory Effects in High Glucose-Treated THP-1 Cells Through Histone Acetyltransferase/Histone Deacetylase Regulation
Arang Kim, Jung-Mi Yun
Journal of Medicinal Food.2017; 20(8): 782. CrossRef - Fucoidan ameliorates pancreatic β‐cell death and impaired insulin synthesis in streptozotocin‐treated β cells and mice via a Sirt‐1‐dependent manner
Wen‐Chun Yu, Yen‐Lin Chen, Pai‐An Hwang, Tso‐Hsiao Chen, Tz‐Chong Chou
Molecular Nutrition & Food Research.2017;[Epub] CrossRef - Effects of microRNA-211 on proliferation and apoptosis of lens epithelial cells by targeting SIRT1 gene in diabetic cataract mice
Kun Zeng, Qi-Gao Feng, Bao-Tao Lin, Da-Hui Ma, Chun-Min Liu
Bioscience Reports.2017;[Epub] CrossRef - Luteolin and fisetin suppress oxidative stress by modulating sirtuins and forkhead box O3a expression under in vitro diabetic conditions
Arang Kim, Wooje Lee, Jung-Mi Yun
Nutrition Research and Practice.2017; 11(5): 430. CrossRef - Melatonin and the pathologies of weakened or dysregulated circadian oscillators
Rüdiger Hardeland
Journal of Pineal Research.2017;[Epub] CrossRef - HSF1 acetylation decreases its transcriptional activity and enhances glucolipotoxicity-induced apoptosis in rat and human beta cells
Indri Purwana, Jun J. Liu, Bernard Portha, Jean Buteau
Diabetologia.2017; 60(8): 1432. CrossRef - Tangshen formula improves inflammation in renal tissue of diabetic nephropathy through SIRT1/NF-κB pathway
Yue‑Guang Du, Ke‑Na Zhang, Zong‑Lei Gao, Fengjiao Dai, Xi‑Xi Wu, Ke‑Fu Chai
Experimental and Therapeutic Medicine.2017;[Epub] CrossRef - MiR-377 promotes white adipose tissue inflammation and decreases insulin sensitivity in obesity via suppression of sirtuin-1 (SIRT1)
Jie Peng, Yinghui Wu, Zhao Deng, Yuanfei Zhou, Tongxing Song, Yang Yang, Xiaming Zhang, Tao Xu, Mao Xia, Anle Cai, Zuhong Liu, Jian Peng
Oncotarget.2017; 8(41): 70550. CrossRef - The role of SIRT1 in diabetic cardiomyopathy
Hedyieh Karbasforooshan, Gholamreza Karimi
Biomedicine & Pharmacotherapy.2017; 90: 386. CrossRef - Long-term obestatin treatment of mice type 2 diabetes increases insulin sensitivity and improves liver function
Paweł A. Kołodziejski, Ewa Pruszyńska-Oszmałek, Mathias Z. Strowski, Krzysztof W. Nowak
Endocrine.2017; 56(3): 538. CrossRef - Overexpressed eNOS upregulates SIRT1 expression and protects mouse pancreatic β cells from apoptosis
Tingting Hu, Ye Chen, Qian Jiang, Jun Lin, Hewei Li, Ping Wang, Leping Feng
Experimental and Therapeutic Medicine.2017; 14(2): 1727. CrossRef - Resveratrol attenuates type 2 diabetes mellitus by mediating mitochondrial biogenesis and lipid metabolism via Sirtuin type 1
Ming‑Ming Cao, Xi Lu, Guo‑Dong Liu, Ying Su, Yan‑Bo Li, Jin Zhou
Experimental and Therapeutic Medicine.2017;[Epub] CrossRef - Resveratrol Improves Glycemic Control in Type 2 Diabetic Obese Mice by Regulating Glucose Transporter Expression in Skeletal Muscle and Liver
Caio Yonamine, Erika Pinheiro-Machado, Maria Michalani, Ana Alves-Wagner, João Esteves, Helayne Freitas, Ubiratan Machado
Molecules.2017; 22(7): 1180. CrossRef - Sleep restriction induced energy, methylation and lipogenesis metabolic switches in rat liver
Arjun Sengupta, Seth D. Rhoades, Eun Ji Kim, Soumyashant Nayak, Gregory R. Grant, Peter Meerlo, Aalim M. Weljie
The International Journal of Biochemistry & Cell Biology.2017; 93: 129. CrossRef - Natural mineral-rich water ingestion by ovariectomized fructose-fed Sprague-Dawley rats: effects on sirtuin 1 and glucocorticoid signaling pathways
Jugal Kishore Das, Milton Severo, Cidália Dionísio Pereira, Emília Patrício, José Magalhães, Rosário Monteiro, Delminda Neves, Maria João Martins
Menopause.2017; 24(5): 563. CrossRef - SIRT1 attenuates neuropathic pain by epigenetic regulation of mGluR1/5 expressions in type 2 diabetic rats
Cheng-Hua Zhou, Ming-Xing Zhang, Sha-Sha Zhou, Huan Li, Jian Gao, Lei Du, Xiao-Xing Yin
Pain.2017; 158(1): 130. CrossRef - MiR-34a contributes to diabetes-related cochlear hair cell apoptosis via SIRT1/HIF-1α signaling
Ying Lin, Jinjin Shen, Danfeng Li, Jie Ming, Xiangyang Liu, Nana Zhang, Jingbo Lai, Min Shi, Qiuhe Ji, Ying Xing
General and Comparative Endocrinology.2017; 246: 63. CrossRef - High glucose upregulates endothelin type B receptors in vascular smooth muscle cells via the downregulation of Sirt1
Yan Lin, Yan Zhao, Enqi Liu
International Journal of Molecular Medicine.2017;[Epub] CrossRef - Anti-diabetic Effects of Ethanol Extract from Bitter Melon in Mice Fed a High-fat Diet
Nal Ae Yoon, Juyeong Park, Jiyeon Lee, Joo Yeon Jeong, Hyun-Kyu Kim, Hak Sung Lee, In Guk Hwang, Gu Seob Roh, Hyun Joon Kim, Gyeong Jae Cho, Wan Sung Choi, Dong Hoon Lee, Sang Soo Kang
Development & Reproduction.2017; 21(3): 259. CrossRef - Thymoquinone ameliorates diabetic phenotype in Diet-Induced Obesity mice via activation of SIRT-1-dependent pathways
Shpetim Karandrea, Huquan Yin, Xiaomei Liang, Angela L. Slitt, Emma A. Heart, Guillermo López Lluch
PLOS ONE.2017; 12(9): e0185374. CrossRef - Artesunate protects pancreatic beta cells against cytokine-induced damage via SIRT1 inhibiting NF-κB activation
L. Yu, J. F. Chen, X. Shuai, Y. Xu, Y. Ding, J. Zhang, W. Yang, X. Liang, D. Su, C. Yan
Journal of Endocrinological Investigation.2016; 39(1): 83. CrossRef - Mir‐217 promotes inflammation and fibrosis in high glucose cultured rat glomerular mesangial cells via Sirt1/HIF‐1α signaling pathway
Ying Shao, Chuan Lv, Can Wu, Yuehong Zhou, Qiuyue Wang
Diabetes/Metabolism Research and Reviews.2016; 32(6): 534. CrossRef - Energetic stress: The reciprocal relationship between energy availability and the stress response
C.S. Harrell, C.F. Gillespie, G.N. Neigh
Physiology & Behavior.2016; 166: 43. CrossRef - Ligand-based virtual screening and inductive learning for identification of SIRT1 inhibitors in natural products
Yunan Sun, Hui Zhou, Hongmei Zhu, Siu-wai Leung
Scientific Reports.2016;[Epub] CrossRef - Modulation of gut microbiota and delayed immunosenescence as a result of syringaresinol consumption in middle-aged mice
Si-Young Cho, Juewon Kim, Ji Hae Lee, Ji Hyun Sim, Dong-Hyun Cho, Il-Hong Bae, Hyunbok Lee, Min A. Seol, Hyun Mu Shin, Tae-Joo Kim, Dae-Yong Kim, Su-Hyung Lee, Song Seok Shin, Sin-Hyeog Im, Hang-Rae Kim
Scientific Reports.2016;[Epub] CrossRef - Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults
Javier Angulo, Mariam El Assar, Leocadio Rodríguez-Mañas
Molecular Aspects of Medicine.2016; 50: 1. CrossRef - Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat
Liqun Ma, Rongguo Fu, Zhaoyang Duan, Jiamei Lu, Jie Gao, Lifang Tian, Zhian Lv, Zhao Chen, Jin Han, Lining Jia, Li Wang
Pathology - Research and Practice.2016; 212(4): 310. CrossRef - The Ticking of the Epigenetic Clock: Antipsychotic Drugs in Old Age
Adonis Sfera, Carolina Osorio, Luzmin Inderias, Michael Cummings
Frontiers in Endocrinology.2016;[Epub] CrossRef - ZLN005 protects cardiomyocytes against high glucose-induced cytotoxicity by promoting SIRT1 expression and autophagy
Wenju Li, Xiaoli Li, Bin Wang, Yan Chen, Aiping Xiao, Di Zeng, Dongbo Ou, Song Yan, Wei Li, Qiangsun Zheng
Experimental Cell Research.2016; 345(1): 25. CrossRef - How much successful are the medicinal chemists in modulation of SIRT1: A critical review
Ashwani Kumar, Shilpi Chauhan
European Journal of Medicinal Chemistry.2016; 119: 45. CrossRef - Network Based Approach in the Establishment of the Relationship between Type 2 Diabetes Mellitus and Its Complications at the Molecular Level Coupled with Molecular Docking Mechanism
Shailima Rampogu, Mary Rampogu Lemuel
BioMed Research International.2016; 2016: 1. CrossRef - 1,4-Dihydropyridines Active on the SIRT1/AMPK Pathway Ameliorate Skin Repair and Mitochondrial Function and Exhibit Inhibition of Proliferation in Cancer Cells
Sergio Valente, Paolo Mellini, Francesco Spallotta, Vincenzo Carafa, Angela Nebbioso, Lucia Polletta, Ilaria Carnevale, Serena Saladini, Daniela Trisciuoglio, Chiara Gabellini, Maria Tardugno, Clemens Zwergel, Chiara Cencioni, Sandra Atlante, Sébastien Mo
Journal of Medicinal Chemistry.2016; 59(4): 1471. CrossRef - Antidiabetic Effects of Resveratrol: The Way Forward in Its Clinical Utility
Omolola R. Oyenihi, Ayodeji B. Oyenihi, Anne A. Adeyanju, Oluwafemi O. Oguntibeju
Journal of Diabetes Research.2016; 2016: 1. CrossRef - Mechanism of uncoupling protein 2-mediated myocardial injury in hypothermic preserved rat hearts
Gai-Ge Chen, Jin-Bin Yan, Xu-Ming Wang, Ming-Zhi Zheng, Jian-Ping Jiang, Xin-Mei Zhou, Bin Cai, Yue-Liang Shen
Molecular Medicine Reports.2016; 14(2): 1857. CrossRef - Identification of sirtuin 1 as a promising therapeutic target for hypertrophic scars
Xiao‐Zhi Bai, Jia‐Qi Liu, Long‐Long Yang, Lei Fan, Ting He, Lin‐Lin Su, Ji‐Hong Shi, Chao‐Wu Tang, Zhao Zheng, Da‐Hai Hu
British Journal of Pharmacology.2016; 173(10): 1589. CrossRef - Physiological regulation of the heat shock response by glutamine: implications for chronic low-grade inflammatory diseases in age-related conditions
Jaqueline Santos Moreira Leite, Vinicius Fernandes Cruzat, Mauricio Krause, Paulo Ivo Homem de Bittencourt
Nutrire.2016;[Epub] CrossRef - In Patients with Coronary Artery Disease and Type 2 Diabetes, SIRT1 Expression in Circulating Mononuclear Cells Is Associated with Levels of Inflammatory Cytokines but Not with Coronary Lesions
Yuanmin Li, Jing Ni, Rong Guo, Weiming Li
BioMed Research International.2016; 2016: 1. CrossRef - The role of bile acids in metabolic regulation
Libor Vítek, Martin Haluzík
Journal of Endocrinology.2016; 228(3): R85. CrossRef - New perspectives on the development of antiobesity drugs
Luca Costantino, Daniela Barlocco
Future Medicinal Chemistry.2015; 7(3): 315. CrossRef - SIRT1 protects against myocardial ischemia–reperfusion injury via activating eNOS in diabetic rats
Mingge Ding, Jingyi Lei, Hongcheng Han, Weibo Li, Yinxian Qu, Enqing Fu, Feng Fu, Xiaoming Wang
Cardiovascular Diabetology.2015;[Epub] CrossRef - Resveratrol and diabetes: from animal to human studies
Tomasz Szkudelski, Katarzyna Szkudelska
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2015; 1852(6): 1145. CrossRef - Resveratrol restores the circadian rhythmic disorder of lipid metabolism induced by high-fat diet in mice
Linjie Sun, Yan Wang, Yu Song, Xiang-Rong Cheng, Shufang Xia, Md Ramim Tanver Rahman, Yonghui Shi, Guowei Le
Biochemical and Biophysical Research Communications.2015; 458(1): 86. CrossRef - DPP‐4 Inhibitor Linagliptin Attenuates Aβ‐induced Cytotoxicity through Activation of AMPK in Neuronal Cells
Edy Kornelius, Chih‐Li Lin, Hsiu‐Han Chang, Hsin‐Hua Li, Wen‐Nung Huang, Yi‐Sun Yang, Ying‐Li Lu, Chiung‐Huei Peng, Chien‐Ning Huang
CNS Neuroscience & Therapeutics.2015; 21(7): 549. CrossRef - Effects of Caloric Intake on Learning and Memory Function in Juvenile C57BL/6J Mice
Bao-Lei Xu, Rong Wang, Li-Na Ma, Wen Dong, Zhi-Wei Zhao, Jing-Shuang Zhang, Yu-Lan Wang, Xu Zhang
BioMed Research International.2015; 2015: 1. CrossRef - Inhibition of microRNA-9-3p reduces lipid accumulation in HepG2 cells by targeting the expression of sirtuin type 1
YA-NAN SUN, SI LI, YUN-TAO ZHOU, JIA LIU, LUO-BING TIAN, YAN-FENG ZHEN, HUI FANG
Molecular Medicine Reports.2015; 12(5): 7742. CrossRef - Bioinformatics analysis of abnormal DNA methylation in muscle samples from monozygotic twins discordant for type 2 diabetes
FEI LIU, QIANQIAN SUN, LINGXIAO WANG, SHUANGSHUANG NIE, JUN LI
Molecular Medicine Reports.2015; 12(1): 351. CrossRef - Resveratrol Protects against Methylglyoxal-Induced Hyperglycemia and Pancreatic Damage In Vivo
An-Sheng Cheng, Yu-Hsiang Cheng, Chi-Ying Lee, Chin-Yuan Chung, Wen-Chang Chang
Nutrients.2015; 7(4): 2850. CrossRef - Ameliorative effects of lutein on non-alcoholic fatty liver disease in rats
Xiang Qiu
World Journal of Gastroenterology.2015; 21(26): 8061. CrossRef - Histone hypoacetylation and increased histone deacetylases in peripheral blood mononuclear cells from patients with Graves' disease
Ni Yan, Jiao-zhen Zhou, Jin-an Zhang, Tiantian Cai, Wen Zhang, Yuan Wang, Fatuma-Said Muhali, Lijuan Guan, Rong-hua Song
Molecular and Cellular Endocrinology.2015; 414: 143. CrossRef - Resveratrol relieves ischemia‑induced oxidative stress in the hippocampus by activating SIRT1
Zhuangzhi Meng, Jianguo Li, Honglin Zhao, Haiying Liu, Guowei Zhang, Lingzhan Wang, He Hu, Di Li, Mingjing Liu, Fulong Bi, Xiaoping Wang, Geng Tian, Qiang Liu, Batu Buren
Experimental and Therapeutic Medicine.2015;[Epub] CrossRef - Could changes in adiponectin drive the effect of statins on the risk of new-onset diabetes? The case of pitavastatin
Lorenzo Arnaboldi, Alberto Corsini
Atherosclerosis Supplements.2015; 16: 1. CrossRef - Obesity-Related Oxidative Stress: the Impact of Physical Activity and Diet Manipulation
Chun-Jung Huang, Matthew J. McAllister, Aaron L. Slusher, Heather E. Webb, J. Thomas Mock, Edmund O. Acevedo
Sports Medicine - Open.2015;[Epub] CrossRef - SIRT1 suppresses cardiomyocyte apoptosis in diabetic cardiomyopathy: An insight into endoplasmic reticulum stress response mechanism
Rong Guo, Weijing Liu, Baoxin Liu, Buchun Zhang, Weiming Li, Yawei Xu
International Journal of Cardiology.2015; 191: 36. CrossRef - Fenofibrate reduces inflammation in obese patients with or without type 2 diabetes mellitus via sirtuin 1/fetuin A axis
Mohamed H. Noureldein, Rania S. Abd El-Razek, Mohamed H. El-Hefnawy, Hala O. El-Mesallamy
Diabetes Research and Clinical Practice.2015; 109(3): 513. CrossRef - Plasma levels of SIRT1 associate with non-alcoholic fatty liver disease in obese patients
Stefania Mariani, Daniela Fiore, Sabrina Basciani, Agnese Persichetti, Savina Contini, Carla Lubrano, Luisa Salvatori, Andrea Lenzi, Lucio Gnessi
Endocrine.2015; 49(3): 711. CrossRef - Platycodon grandiflorum A. De Candolle Ethanolic Extract Inhibits Adipogenic Regulators in 3T3-L1 Cells and Induces Mitochondrial Biogenesis in Primary Brown Preadipocytes
Hye-Lin Kim, Jinbong Park, Hyewon Park, Yunu Jung, Dong-Hyun Youn, JongWook Kang, Mi-Young Jeong, Jae-Young Um
Journal of Agricultural and Food Chemistry.2015; 63(35): 7721. CrossRef - Amazonian Fruits: An Overview of Nutrients, Calories and Use in Metabolic Disorders
Moacir Couto de Andrade Júnior, Jerusa Souza Andrade
Food and Nutrition Sciences.2014; 05(17): 1692. CrossRef - Protective effects of resveratrol on postmenopausal osteoporosis: regulation of SIRT1-NF-κB signaling pathway
Jing Feng, Shuai Liu, Sai Ma, Jian Zhao, Wei Zhang, Wei Qi, Pengchong Cao, Zheng Wang, Wei Lei
Acta Biochimica et Biophysica Sinica.2014; 46(12): 1024. CrossRef - The fat cell senescence hypothesis
Philip Newsholme, Paulo I. Homem de Bittencourt
Current Opinion in Clinical Nutrition and Metabolic Care.2014; 17(4): 295. CrossRef - Sirt1 and Sirt6 Mediate Beneficial Effects of Rosiglitazone on Hepatic Lipid Accumulation
Soo Jin Yang, Jung Mook Choi, Eugene Chang, Sung Woo Park, Cheol-Young Park, Aimin Xu
PLoS ONE.2014; 9(8): e105456. CrossRef - Resveratrol ameliorates hepatic metaflammation and inhibits NLRP3 inflammasome activation
Soo Jin Yang, Yunsook Lim
Metabolism.2014; 63(5): 693. CrossRef - Role of SIRT1 in Streptococcus pneumoniae-induced human β-defensin-2 and interleukin-8 expression in A549 cell
Li Lin, Shun-hang Wen, Shu-zhen Guo, Xiao-yan Su, Hu-jun Wu, Lei Chong, Hai-lin Zhang, Wei-xi Zhang, Chang-chong Li
Molecular and Cellular Biochemistry.2014; 394(1-2): 199. CrossRef

 KDA
KDA PubReader
PubReader Cite
Cite




