
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 44(5); 2020 > Article
-
Original ArticleMetabolic Risk/Epidemiology A Comparison of Predictive Performances between Old versus New Criteria in a Risk-Based Screening Strategy for Gestational Diabetes Mellitus
-
Subeen Hong1,2*
 , Seung Mi Lee1*
, Seung Mi Lee1* , Soo Heon Kwak3, Byoung Jae Kim1,4, Ja Nam Koo5, Ig Hwan Oh5, Sohee Oh6, Sun Min Kim1,4, Sue Shin7,8, Won Kim3,9, Sae Kyung Joo3,9, Errol R. Norwitz10, Souphaphone Louangsenlath11, Chan-Wook Park1, Jong Kwan Jun1, Joong Shin Park1
, Soo Heon Kwak3, Byoung Jae Kim1,4, Ja Nam Koo5, Ig Hwan Oh5, Sohee Oh6, Sun Min Kim1,4, Sue Shin7,8, Won Kim3,9, Sae Kyung Joo3,9, Errol R. Norwitz10, Souphaphone Louangsenlath11, Chan-Wook Park1, Jong Kwan Jun1, Joong Shin Park1
-
Diabetes & Metabolism Journal 2020;44(5):726-736.
DOI: https://doi.org/10.4093/dmj.2019.0126
Published online: April 13, 2020
1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea
2Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
4Department of Obstetrics and Gynecology, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea
5Department of Obstetrics and Gynecology, Seoul Women's Hospital, Incheon, Korea
6Department of Biostatistics, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea
7Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea
8Department of Laboratory Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea
9Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea
10Department of Obstetrics and Gynecology, Tufts University School of Medicine, Boston, MA, USA
11Department of Obstetrics and Gynecology, University of Health Science, Vientiane, Laos
- Corresponding author: Corresponding author: Joong Shin Park. Department of Obstetrics and Gynecology, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea. jsparkmd@snu.ac.kr
- *Subeen Hong and Seung Mi Lee contributed equally to this study as first authors.
Copyright © 2020 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- The definition of the high-risk group for gestational diabetes mellitus (GDM) defined by the American College of Obstetricians and Gynecologists was changed from the criteria composed of five historic/demographic factors (old criteria) to the criteria consisting of 11 factors (new criteria) in 2017. To compare the predictive performances between these two sets of criteria.
-
Methods
- This is a secondary analysis of a large prospective cohort study of non-diabetic Korean women with singleton pregnancies designed to examine the risk of GDM in women with nonalcoholic fatty liver disease. Maternal fasting blood was taken at 10 to 14 weeks of gestation and measured for glucose and lipid parameters. GDM was diagnosed by the two-step approach.
-
Results
- Among 820 women, 42 (5.1%) were diagnosed with GDM. Using the old criteria, 29.8% (n=244) of women would have been identified as high risk versus 16.0% (n=131) using the new criteria. Of the 42 women who developed GDM, 45.2% (n=19) would have been mislabeled as not high risk by the old criteria versus 50.0% (n=21) using the new criteria (1-sensitivity, 45.2% vs. 50.0%, P>0.05). Among the 778 patients who did not develop GDM, 28.4% (n=221) would have been identified as high risk using the old criteria versus 14.1% (n=110) using the new criteria (1-specificity, 28.4% vs. 14.1%, P<0.001).
-
Conclusion
- Compared with the old criteria, use of the new criteria would have decreased the number of patients identified as high risk and thus requiring early GDM screening by half (from 244 [29.8%] to 131 [16.0%]).
- Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance that is first recognized during pregnancy [1]. GDM is one of the most common complications during pregnancy, with a reported prevalence of 5.7% to 9.5% in pregnant Korean women [23]. GDM is related to not only maternal complications but also fetal/neonatal adverse outcomes; therefore, the diagnosis of GDM in the appropriate period and adequate glucose control are helpful to minimize these complications[456].
- In the 4th International Workshop Conference on GDM in 1998, classifying pregnant women according to the risk for GDM into low-, intermediate-, and high-risk groups and determining a differential screening strategy for each risk group were recommended. The high-risk group was defined as those with maternal demographic risk factors (i.e., strong family history, marked obesity, history of GDM, glucose intolerance, and glucosuria), and a glucose tolerance test at their first prenatal visit was recommended for this high-risk group [7]. These criteria for the high-risk group were reaffirmed at the 5th International Workshop Conference in 2005 and have been also used in the clinical guidelines of the American College of Obstetricians and Gynecologists (ACOG) [89]. Until now, the clinical effectiveness of the criteria for the high-risk group has not been well evaluated in previous studies, although this strategy has been widely implemented in clinical practice [1011121314].
- Otherwise, the American Diabetes Association (ADA) recommended in 2012 early testing for diabetes at the first prenatal visit in women with risk factors for type 2 diabetes mellitus [15]. Moreover, the new criteria for pregnant women was exactly derived from the criteria in non-pregnant adults for high risk of diabetes, because of increasing prevalence of type 2 diabetes mellitus in women of child-bearing age [16]. Therefore, the criteria for high risk for GDM suggested in this recommendation were different from those in the ACOG guidelines, consisting of 11 clinical factors (Supplementary Fig. 1). In 2017, ACOG also adopted this ADA recommendation and recommended early screening for GDM according to these new criteria [1]. Unlike criteria of the high-risk population in 5th International Workshop on GDM (old criteria), the criteria of the high-risk population by the ADA (new criteria) includes the degree of obesity and laboratory results [817].
- In Korea, the screening strategy for the high-risk group and diagnosis of GDM has been conducted based on the ACOG guidelines. Although the acceptance of new criteria is an issue of paramount importance in clinical practice, these two criteria have not been compared in terms of their ability to predict the development of GDM until now. Furthermore, because some risk factors such as low high density lipoprotein cholesterol (HDL-C) and previous history of cardiovascular disease which are included in the new criteria are rare in young women, it is necessary to verify their significance for predicting the development of GDM. In this study, we compared the predictive performance for detecting GDM between the old and new criteria.
INTRODUCTION
- Ethics
- The current study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. 1810-047-977). Written informed consent was obtained from all participants at the time of enrollment of the original study.
- Study design
- This study is a secondary analysis of the ongoing large prospective cohort study designed to examine the risk of GDM in women with nonalcoholic fatty liver disease (“Fatty Liver in Pregnancy” registry, NCT02276144) [18]. The subjects of this study are non-diabetic Korean women with singleton pregnancy whose data contains the information for assessing clinical and demographic risk by both the old and new criteria and the results of the diagnostic tests for GDM during pregnancy. The predictive ability of these risk criteria for the development of GDM was compared between the old and new criteria.
- The setting of a prospective cohort study
- In 2014, there had been a large prospective cohort study of nonalcoholic fatty liver disease in pregnancy conducted in three centers (Incheon Seoul Women Hospital, Seoul Metropolitan Government Seoul National University Boramae Medical Center, and Seoul National University Hospital) in South Korea to examine the risk of GDM in women with nonalcoholic fatty liver disease. Incheon Seoul Women Hospital, as the primary obstetric care center, has approximately 4,000 deliveries annually; and Seoul National University Boramae Medical Center, as a referral center, has approximately 500 deliveries annually. Participants were recruited at these two hospitals, and investigators at Seoul National University Hospital designed the study protocol and analyzed data. The protocol of the original research is detailed in the previous report [18].
- Study population of the current study
- The women enrolled from October 2014 to October 2017 were included in the current study. All participants visiting antenatal care centers before 14 weeks of gestation were enrolled after obtaining informed consent. Women who agreed to secondary analysis and who completed diagnostic tests (two-step approach) for GDM were included. Women with pre-gestational diabetes mellitus or who wanted to withdraw from the study were excluded. Among them, the eligible study population fulfilled all of the data of clinical/demographic risk factors in old and new criteria. Cases with no information about at least one of the risk factors in the old and new criteria were excluded to compare sensitivity and specificity between old and new criteria.
- The definition and evaluation of risk factors of GDM
- The presence of each risk factor included in the old or new criteria was evaluated in the study population. Among the risk factors, clinical characteristics including pre-pregnancy body mass index (BMI), family history of diabetes, history of gestational diabetes in prior pregnancy, maternal underlying disease such as pre-pregnancy diabetes, hypertension, and cardiovascular disease were collected routinely at the time of enrollment. At 10 to 14 weeks of gestation, the degree of physical activity was also evaluated by The International Physical Activity Questionnaire [19], and fasting blood samples after an 8-hour fasting were collected at the time of liver ultrasound (which was conducted for the original cohort study) for measurement of fasting glucose and lipid parameters such as triglyceride (TG) and HDL-C. In addition, the presence of glucosuria in early pregnancy, the delivery history of macrosomia, and the diagnosis of polycystic ovarian syndrome (PCOS) before pregnancy were evaluated by review of medical records. The presence of glucosuria is routinely evaluated in early pregnancy in our institutions.
- For BMI classification, World Health Organization criteria for an Asian population were adopted, because the study population consisted of only Korean pregnant women [2021]. Overweight and obese was defined as BMI ≥23 and ≥25 kg/m2, respectively, and severe obesity was defined as BMI ≥30 kg/m2, which are the suggested criteria for obesity class II (severe obesity) in an Asian population. Glucosuria was defined as +1 or more a dipstick at urinary analysis in early pregnancy [22]. Physical inactivity was defined as no leisure time physical activity in the last 7 days [23]. Impaired glucose metabolism was defined as fasting blood glucose level of ≥100 mg/dL [24]. Other criteria of impaired glucose metabolism (glycosylated hemoglobin and impaired glucose tolerance) were not available in the current study.
- Diagnosis of GDM
- GDM was diagnosed by the two-step approach, a 50 g screening glucose tolerance test (OGTT) followed by a diagnostic 100 g OGTT according to the ACOG guidelines [1]. Women with measured plasma glucose level ≥140 mg/dL at 50 g OGTT were examined for 100 g OGTT. A diagnosis for GDM required two or more elevated glucose values in 100 g OGTT with the cut-off values of the Carpenter and Coustan thresholds (95 mg/dL for fasting glucose, 180 mg/dL for 1-hour glucose, 155 mg/dL for 2-hour glucose, and >140 mg/dL for 3-hour glucose) [25].
- Statistical analysis
- Continuous variables were described by median and interquartile range, and categorical variables were described by numbers and percentage. The comparison of continuous variables was performed using the independent t-test or the Mann-Whitney U test. Categorical variable were compared with the chi-square test or the Fisher exact test, where appropriate. Using univariable logistic regression analysis, odds ratios (ORs) and 95% confidential intervals (CIs) of risk factors for GDM were evaluated. For determining the independent risk factors, multivariable logistic regression analysis was conducted using variables chosen with a P value of <0.05 in the univariable analysis with backward elimination. In the multivariable logistic regression, Firth's penalized likelihood bias reduction was used to avoid bias in parameter estimates due to the small sample size [26]. To compare predictive performance such as detection rate and false-positive rate between the old and new criteria, the McNemar test was applied. Missing data were treated as missing observations. A P value of <0.05 was considered statistically significant. IBM SPSS Statistics version 23.0 software (IBM Inc., Armonk, NY, USA) and R version 3.5.1 (http://www.r-project.org) were used for the analyses.
METHODS
- Study population
- During the study period, a total of 1,077 women without pre-gestational diabetes were recruited between October 2014 and October 2017 and completed the test for GDM. Among these women, 257 subjects (132 women who did not have a fasting blood sample at 10 to 14 weeks of gestation, three women who did not report their degree of physical activity, 36 women without data on glucosuria, 23 women without data on the history of PCOS, and 63 women without data on history of macrosomia in a previous pregnancy) were excluded from the final analysis.
- In 820 women in the final study population, 42 (5.1%) women were diagnosed with GDM and 12 (1.5%) women with GDM were managed on insulin (Fig. 1). Among 820 women, 29.8% (n=244) of women would have been identified as high risk using the old criteria, whereas 16.0% (n=131) would have been identified as high risk using the new criteria. Among 244 women who were assessed as high risk by the old criteria, 9.5% (n= 23) of women were diagnosed with GDM and 3.3% (n=8) of women were managed on insulin. Among the 131 women who were assessed as high risk by the new criteria, 16.0% (n=21) of women were diagnosed with GDM and 6.1% (n=8) of women were managed on insulin.
- Basal characteristics and obstetric outcome according to the presence of GDM
- Basal characteristics and obstetric outcome of the study population according to the GDM status are presented in the Supplementary Table 1. The median maternal age and the frequency of nulliparity were not different between the two groups. Women who developed GDM had a higher median pre-pregnancy BMI and a higher rate of previous history of GDM and chronic hypertension. The gestational age at delivery, birth weight, and the risk of macrosomia or cesarean delivery were not different between the two groups. Women with GDM were more likely to have large for gestational age neonates, but this difference did not reach statistical significance.
- Odds ratio of risk factors for GDM
- Table 1 presents the OR of individual risk factors consisting of the old or new criteria for high risk of GDM. BMI ≥23 kg/m2, first-degree relative with diabetes, chronic hypertension, previous history of GDM, impaired fasting glucose, and TG >250 mg/dL were associated with the development of GDM. Similarly, BMI ≥23 kg/m2, chronic hypertension, previous history of GDM, glucosuria, impaired fasting glucose, HDL-C <35 mg/dL, and TG >250 mg/dL were associated with the development of GDM on insulin. However, PCOS history, physical inactivity, previous infant with macrosomia, glucosuria, and HDL-C <35 mg/dL were not related to the risk of GDM.
- Odds ratio of risk factors by old and new criteria
- Table 2 shows that all of the risk factors in the old criteria were significantly associated with the development of GDM, except glucosuria. Overall, the OR of the high-risk group for GDM by the old criteria was 3.05 (95% CI, 1.63 to 5.71). By the new criteria, overweight women who had one of the risk factors such as first-degree relative with diabetes, previous GDM, chronic hypertension, TG >250 mg/dL, impaired fasting glucose, or severe obesity increased the risk of the development of GDM significantly. Overall, the OR of the high-risk group by the new criteria was 6.07 (95% CI, 3.21 to 11.49), higher than those by the old criteria.
- Table 3 also presents the OR of individual risk factors for GDM requiring insulin treatment according to the old or new criteria. The OR of the high-risk group by the old criteria and the new criteria was 5.04 (95% CI, 1.50 to 16.91) and 12.15 (95% CI, 3.60 to 41.02), respectively.
- Predictive performance of old vs. new criteria
- As shown in Table 4, the detection rate and false-positive rate were compared between the two criteria. Of the 42 women who developed GDM, the old criteria would have classified 54.8% of women as high risk, whereas the new criteria would have classified 50% of women as high risk (P>0.05). Among the 778 patients who did not develop GDM, 28.4% (n=221) would have been identified as high risk using the old criteria versus 14.1% (n=110) using the new criteria (P<0.001). For prediction of GDM requiring insulin treatment, the detection rate was 66.7% for both criteria, and the false-positive rate was lower when using the new criteria than the old criteria (29.2% vs. 15.2%, P<0.001).
- Independent risk factors for GDM
- Table 5 shows multivariable logistic regression analysis conducted to determine independent risk factors for GDM. Among various risk factors consisting of old or new criteria, only four factors (BMI, previous gestational diabetes, TG >250 mg/dL, and fasting blood gluocose ≥100 mg/dL) were independent risk factors.
RESULTS
- Principal findings of the study
- (1) The prevalence of GDM and GDM managed on insulin was 5.1% and 1.5%, respectively. (2) Compared with the old criteria, the use of the new criteria would have decreased the number of patients identified as high risk and thus requiring early GDM screening by half (from 29.8% to 16.0%). (3) Although the detection rate for GDM was similar between the two criteria, the false-positive rate was significantly lower in the new criteria compared with the old criteria. (4) Among the suggested risk factors, only BMI, previous gestational diabetes, TG >250 mg/dL, and impaired fasting glucose were independent risk factors.
- High-risk population for GDM
- There has been so much effort to establish criteria by which the high-risk group of GDM can be classified, because the number of pregnant women who are examined with unnecessary screening tests could be reduced. Previous studies had researched validating the performances of the risk-based screening guidelines or scoring systems of GDM [272829]. According to the current systematic review study evaluating the association of risk factors with GDM, it was not possible to offer pregnant women gold standard screening methods for detecting of GDM [30]. To this day, the criteria for the high-risk group of GDM used in each country are not unified [153132]. The aim of the present study was to investigate which criteria had better predictive performance for developing GDM between the old and new criteria adopted by ACOG. Although the acceptance of the new criteria is an issue of paramount importance in clinical practice, the comparison between the old and new criteria has not been evaluated in the literature. To our knowledge, our study is the first report that compares predictive performances of the old and new high-risk criteria for GDM. In the current study, the detection rate of the new criteria is similar to the old criteria, but the false-positive rate is lower in the new criteria than in the old criteria. According to these findings, fewer people are classified as high risk and, thus, do not receive unnecessary screening tests. However, pregnant women should have their laboratory results such as TG and HDL-C level for their risk assessment by the new criteria. Therefore, for applying the new criteria in the clinical setting, cost-effective analysis is necessary. Moreover, both criteria missed around half of patients (45% vs. 50%) who subsequently developed GDM. More studies are needed to confirm the clinical utility of using the new criteria.
- Independent risk factors for GDM
- Among the new criteria risk factors for GDM, physical inactivity, macrosomia history, low HDL-C, and PCOS were not significant risk factors for GDM. After analyzing multivariable logistic regression, only four factors, including BMI ≥25 kg/m2, previous gestational diabetes, TG >250 mg/dL, and fasting blood gluocose ≥100 mg/dL were independent risk factors for GDM. There have been previous studies evaluating predictable markers for GDM using a maternal blood sample in early pregnancy. Elevated fasting glucose level in early pregnancy has been well known as a risk factor of GDM [333435]. The previous studies about the relationship of lipid concentrations in early pregnancy and GDM showed that only elevated triglyceride is significantly associated with GDM, whereas other lipids are not [3637]. These results are consistent with our findings. Thus, evaluating level of TG and fasting blood glucose at the early pregnancy visit might be clinically useful for predicting GDM. And, these findings are expected to help build a new model for GDM prediction. The determination of the clinical value of each risk factor is also needed in large study populations. Further studies regarding an optimal classification system of risk-based GDM screening are also needed.
- Strength and limitation
- This is the first study validating both the new and old criteria adopted by ACOG. According to the study protocol, which was designed to determine the risk of GDM in patients with nonalcoholic fatty liver disease, we prospectively collected the clinical factors that are known as risk factors for GDM, such as previous history of GDM and family history of diabetes. In addition, we also collected a fasting blood sample at 10 to 14 weeks of gestation, and measured HDL-C, TG, and glucose in these blood samples. This prospective collection of clinical data and laboratory results allowed accurate determination of the ability of the old and new criteria to predict GDM. It is very different from previous studies validating risk-based screening strategies. In addition, the current study evaluated the risk factors for GDM in an Asian population. As the frequency or risk factors for diabetes may be different among races or ethnicities, it is necessary to evaluate the effectiveness of the risk-based GDM screening strategies in an Asian population. Until now, there had been no research about risk-based screening strategies for GDM in Asian countries. Compared to the previous meta-analysis analyzed risk factors for GDM in Asia, the prevalence of GDM and the distributions of risk factors of GDM are similar to that of the subjects of our study [38]. We expect that our study provides clinical information of risk-based screening strategy to other Asian countries.
- There are several points to be considered. First, we evaluated the false-positive rate and detection rate for GDM diagnosed during any period of gestation, although the high-risk criteria targets selection of the high-risk group for GDM diagnosed early in pregnancy or pre-gestational diabetes. Second, the criteria with laboratory results are based on the result of the blood taken at 10 to 14 weeks of gestation. The optimal blood testing period for judgment of high risk (i.e., pre-gestational blood test vs. blood test in early pregnancy) is not clear in the guidelines. Third, the number of patients with GDM and GDM on insulin is insufficient to show a significant difference in sensitivity between old and new criteria.
- Further study
- To confirm the clinical utility of the new criteria or selective risk-based screening for early GDM, more prospective studies and randomized controlled trials will be needed comparing outcome between populations managed according to the strategy and not. To suggest the appropriate screening strategy for GDM, comparison and validation of various screening strategies are also needed. As these criteria were originally designed to select the high-risk group at the first prenatal visit, further research is needed to determine the performances of these criteria for detecting early GDM.
- Conclusion
- Compared with the old criteria, use of the new criteria would have decreased the number of patients identified as high risk and, thus, reduced early GDM screening by half. Similarly, the use of the new criteria would have decreased by half the number of patients who did not develop GDM from having to undergo early screening. More studies are needed to confirm the clinical utility of using the new ADA criteria.
DISCUSSION
-
Acknowledgements
- This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science and ICT of Korea (2016M3A9B6902061). Funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: S.H., S.M.L., S.H.K., J.S.P.
Acquisition, analysis, or interpretation of data: S.H., S.M.L., S.H.K., B.J.K., J.N.K., I.H.O., S.O., S.M.K., S.S., W.K., S.K.J., S.L., C.W.P., J.K.J.
Drafting the work or revising: S.H., S.M.L., S.O., E.R.N.
Final approval of the manuscript: J.S.P.
NOTES
SUPPLEMENTARY MATERIALS
Supplementary Fig. 1
- 1. Committee on Practice Bulletins: Obstetrics. Practice bulletin no. 180: gestational diabetes mellitus. Obstet Gynecol 2017;130:e17-e37.ArticlePubMed
- 2. Koo BK, Lee JH, Kim J, Jang EJ, Lee CH. Prevalence of gestational diabetes mellitus in Korea: a National Health Insurance Database Study. PLoS One 2016;11:e0153107.ArticlePubMedPMC
- 3. Nguyen CL, Pham NM, Binns CW, Duong DV, Lee AH. Prevalence of gestational diabetes mellitus in Eastern and Southeastern Asia: a systematic review and meta-analysis. J Diabetes Res 2018;2018:6536974.ArticlePubMedPMCPDF
- 4. Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, Lowe LP, Trimble ER, Coustan DR, Hadden DR, Persson B, Hod M, Oats JJ. HAPO Study Cooperative Research Group. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012;35:780-786.PubMedPMC
- 5. HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991-2002.ArticlePubMed
- 6. Gonzalez-Quintero VH, Istwan NB, Rhea DJ, Rodriguez LI, Cotter A, Carter J, Mueller A, Stanziano GJ. The impact of glycemic control on neonatal outcome in singleton pregnancies complicated by gestational diabetes. Diabetes Care 2007;30:467-470.ArticlePubMedPDF
- 7. Metzger BE, Coustan DR. Summary and recommendations of the fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care 1998;21 Suppl 2:B161-B167.PubMed
- 8. Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, Hod M, Kitzmiller JL, Kjos SL, Oats JN, Pettitt DJ, Sacks DA, Zoupas C. Summary and recommendations of the fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30 Suppl 2:S251-S260.ArticlePubMedPDF
- 9. Committee on Practice Bulletins: Obstetrics. Practice bulletin no. 137: gestational diabetes mellitus. Obstet Gynecol 2013;122(2 Pt 1):406-416.PubMed
- 10. Moyer VA. U.S. Preventive Services Task Force. Screening for gestational diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:414-420.ArticlePubMed
- 11. Danilenko-Dixon DR, van Winter JT, Nelson RL, Ogburn PL Jr. Universal versus selective gestational diabetes screening: application of 1997 American Diabetes Association recommendations. Am J Obstet Gynecol 1999;181:798-802.ArticlePubMed
- 12. Cosson E, Benchimol M, Carbillon L, Pharisien I, Paries J, Valensi P, Lormeau B, Bolie S, Uzan M, Attali JR. Universal rather than selective screening for gestational diabetes mellitus may improve fetal outcomes. Diabetes Metab 2006;32:140-146.ArticlePubMed
- 13. Cosson E, Benbara A, Pharisien I, Nguyen MT, Revaux A, Lormeau B, Sandre-Banon D, Assad N, Pillegand C, Valensi P, Carbillon L. Diagnostic and prognostic performances over 9 years of a selective screening strategy for gestational diabetes mellitus in a cohort of 18,775 subjects. Diabetes Care 2013;36:598-603.ArticlePubMedPMCPDF
- 14. Hieronimus S, Le Meaux JP. Relevance of gestational diabetes mellitus screening and comparison of selective with universal strategies. Diabetes Metab 2010;36(6 Pt 2):575-586.ArticlePubMed
- 15. American Diabetes Association. Standards of medical care in diabetes: 2012. Diabetes Care 2012;35:Suppl 1. S11-S63.ArticlePubMedPDF
- 16. Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999-2005. Diabetes Care 2008;31:899-904.ArticlePubMedPDF
- 17. American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2017;40:Suppl 1. S11-S24.ArticlePubMedPDF
- 18. Lee SM, Kwak SH, Koo JN, Oh IH, Kwon JE, Kim BJ, Kim SM, Kim SY, Kim GM, Joo SK, Koo BK, Shin S, Vixay C, Norwitz ER, Park CW, Jun JK, Kim W, Park JS. Non-alcoholic fatty liver disease in the first trimester and subsequent development of gestational diabetes mellitus. Diabetologia 2019;62:238-248.ArticlePubMedPDF
- 19. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381-1395.ArticlePubMed
- 20. World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000.
- 21. Kim Y, Suh YK, Choi H. BMI and metabolic disorders in South Korean adults: 1998 Korea National Health and Nutrition Survey. Obes Res 2004;12:445-453.ArticlePubMed
- 22. Poyhonen-Alho MK, Teramo KA, Kaaja RJ, Hiilesmaa VK. 50gram oral glucose challenge test combined with risk factor-based screening for gestational diabetes. Eur J Obstet Gynecol Reprod Biol 2005;121:34-37.ArticlePubMed
- 23. Yadav K, Krishnan A. Changing patterns of diet, physical activity and obesity among urban, rural and slum populations in north India. Obes Rev 2008;9:400-408.ArticlePubMed
- 24. Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B. American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753-759.PubMed
- 25. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982;144:768-773.ArticlePubMed
- 26. Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993;80:27-38.ArticlePDF
- 27. Teh WT, Teede HJ, Paul E, Harrison CL, Wallace EM, Allan C. Risk factors for gestational diabetes mellitus: implications for the application of screening guidelines. Aust N Z J Obstet Gynaecol 2011;51:26-30.ArticlePubMed
- 28. Avalos GE, Owens LA, Dunne F. ATLANTIC DIP Collaborators. Applying current screening tools for gestational diabetes mellitus to a European population: is it time for change. Diabetes Care 2013;36:3040-3044.ArticlePubMedPMCPDF
- 29. Syngelaki A, Pastides A, Kotecha R, Wright A, Akolekar R, Nicolaides KH. First-trimester screening for gestational diabetes mellitus based on maternal characteristics and history. Fetal Diagn Ther 2015;38:14-21.ArticlePubMedPDF
- 30. Farrar D, Simmonds M, Bryant M, Lawlor DA, Dunne F, Tuffnell D, Sheldon TA. Risk factor screening to identify women requiring oral glucose tolerance testing to diagnose gestational diabetes: a systematic review and meta-analysis and analysis of two pregnancy cohorts. PLoS One 2017;12:e0175288.ArticlePubMedPMC
- 31. National Collaborating Centre for Women's and Children's Health (UK). Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. London: National Institute for Health and Care Excellence (UK); 2015.
- 32. Nankervis A, McIntyre HD, Moses R, Ross GP, Callaway L, Porter C, Jeffries W, Boorman C, De Vries B, McElduff A. the Australasian Diabetes in Pregnancy Society. ADIPS consensus guidelines for the testing and diagnosis of hyperglycaemia in pregnancy in Australia and New Zealand. Sydney: Australasian Diabetes in Pregnancy Society; 2014.
- 33. Riskin-Mashiah S, Younes G, Damti A, Auslender R. First-trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care 2009;32:1639-1643.ArticlePubMedPMCPDF
- 34. Zhu WW, Yang HX, Wei YM, Yan J, Wang ZL, Li XL, Wu HR, Li N, Zhang MH, Liu XH, Zhang H, Wang YH, Niu JM, Gan YJ, Zhong LR, Wang YF, Kapur A. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. Diabetes Care 2013;36:586-590.ArticlePubMedPMCPDF
- 35. Riskin-Mashiah S, Damti A, Younes G, Auslender R. First trimester fasting hyperglycemia as a predictor for the development of gestational diabetes mellitus. Eur J Obstet Gynecol Reprod Biol 2010;152:163-167.ArticlePubMed
- 36. Enquobahrie DA, Williams MA, Qiu C, Luthy DA. Early pregnancy lipid concentrations and the risk of gestational diabetes mellitus. Diabetes Res Clin Pract 2005;70:134-142.ArticlePubMed
- 37. Li G, Kong L, Zhang L, Fan L, Su Y, Rose JC, Zhang W. Early pregnancy maternal lipid profiles and the risk of gestational diabetes mellitus stratified for body mass index. Reprod Sci 2015;22:712-717.ArticlePubMedPMCPDF
- 38. Lee KW, Ching SM, Ramachandran V, Yee A, Hoo FK, Chia YC, Wan Sulaiman WA, Suppiah S, Mohamed MH, Veettil SK. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2018;18:494.ArticlePubMedPMCPDF
REFERENCES
| Variable | No. of high risk | Detection rate, % | P valuea | False-positive rate, % | P valuea |
|---|---|---|---|---|---|
| For GDM | |||||
| Old criteria | 244 (29.8) | 54.8b | - | 28.4c | - |
| New criteria | 131 (16.0) | 50.0b | 0.754 | 14.1c | <0.001 |
| For GDM on insulin | |||||
| Old criteria | 244 (29.8) | 66.7d | - | 29.2e | - |
| New criteria | 131 (16.0) | 66.7d | >0.999 | 15.2e | <0.001 |
Values are presented as number (%).
GDM, gestational diabetes mellitus.
a P values are for the comparison of new criteria with old criteria,
b Values are based on a total of 42 women with GDM,
c Values are based on a total of 778 women without GDM,
d Values are based on a total of 12 women with GDM on insulin,
e Values are based on a total of 808 women without GDM on insulin.
Multivariable logistic regression analysis is conducted using variables chosen with a P value of <0.05 in the univariable analysis with backward elimination (BMI, previous gestational diabetes, first-degree relative with diabetes, chronic hypertension, TG >250 mg/dL, and impaired fasting glucose).
OR, odds ratio; CI, confidence interval; BMI, body mass index; GDM, gestational diabetes mellitus; TG, triglyceride; FBS, fasting blood glucose.
Figure & Data
References
Citations

- Predicting the Risk of Insulin-Requiring Gestational Diabetes before Pregnancy: A Model Generated from a Nationwide Population-Based Cohort Study in Korea
Seung-Hwan Lee, Jin Yu, Kyungdo Han, Seung Woo Lee, Sang Youn You, Hun-Sung Kim, Jae-Hyoung Cho, Kun-Ho Yoon, Mee Kyoung Kim
Endocrinology and Metabolism.2023; 38(1): 129. CrossRef - Metabolic Dysfunction-Associated Fatty Liver Disease and Subsequent Development of Adverse Pregnancy Outcomes
Seung Mi Lee, Young Mi Jung, Eun Saem Choi, Soo Heon Kwak, Ja Nam Koo, Ig Hwan Oh, Byoung Jae Kim, Sun Min Kim, Sang Youn Kim, Gyoung Min Kim, Sae Kyung Joo, Bo Kyung Koo, Sue Shin, Errol R. Norwitz, Chan-Wook Park, Jong Kwan Jun, Won Kim, Joong Shin Park
Clinical Gastroenterology and Hepatology.2022; 20(11): 2542. CrossRef - Nonalcoholic fatty liver disease and early prediction of gestational diabetes mellitus using machine learning methods
Seung Mi Lee, Suhyun Hwangbo, Errol R. Norwitz, Ja Nam Koo, Ig Hwan Oh, Eun Saem Choi, Young Mi Jung, Sun Min Kim, Byoung Jae Kim, Sang Youn Kim, Gyoung Min Kim, Won Kim, Sae Kyung Joo, Sue Shin, Chan-Wook Park, Taesung Park, Joong Shin Park
Clinical and Molecular Hepatology.2022; 28(1): 105. CrossRef - Nonalcoholic fatty liver disease-based risk prediction of adverse pregnancy outcomes: Ready for prime time?
Seung Mi Lee, Won Kim
Clinical and Molecular Hepatology.2022; 28(1): 47. CrossRef - Postprandial Free Fatty Acids at Mid-Pregnancy Increase the Risk of Large-for-Gestational-Age Newborns in Women with Gestational Diabetes Mellitus
So-Yeon Kim, Young Shin Song, Soo-Kyung Kim, Yong-Wook Cho, Kyung-Soo Kim
Diabetes & Metabolism Journal.2022; 46(1): 140. CrossRef - Effect of Different Types of Diagnostic Criteria for Gestational Diabetes Mellitus on Adverse Neonatal Outcomes: A Systematic Review, Meta-Analysis, and Meta-Regression
Fahimeh Ramezani Tehrani, Marzieh Saei Ghare Naz, Razieh Bidhendi-Yarandi, Samira Behboudi-Gandevani
Diabetes & Metabolism Journal.2022; 46(4): 605. CrossRef - Development of early prediction model for pregnancy-associated hypertension with graph-based semi-supervised learning
Seung Mi Lee, Yonghyun Nam, Eun Saem Choi, Young Mi Jung, Vivek Sriram, Jacob S. Leiby, Ja Nam Koo, Ig Hwan Oh, Byoung Jae Kim, Sun Min Kim, Sang Youn Kim, Gyoung Min Kim, Sae Kyung Joo, Sue Shin, Errol R. Norwitz, Chan-Wook Park, Jong Kwan Jun, Won Kim,
Scientific Reports.2022;[Epub] CrossRef - The Clinical Characteristics of Gestational Diabetes Mellitus in Korea: A National Health Information Database Study
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
Endocrinology and Metabolism.2021; 36(3): 628. CrossRef - The risk of pregnancy‐associated hypertension in women with nonalcoholic fatty liver disease
Young Mi Jung, Seung Mi Lee, Subeen Hong, Ja Nam Koo, Ig Hwan Oh, Byoung Jae Kim, Sun Min Kim, Sang Youn Kim, Gyoung Min Kim, Sae Kyung Joo, Sue Shin, Errol R. Norwitz, Chan‐Wook Park, Jong Kwan Jun, Won Kim, Joong Shin Park
Liver International.2020; 40(10): 2417. CrossRef

 KDA
KDA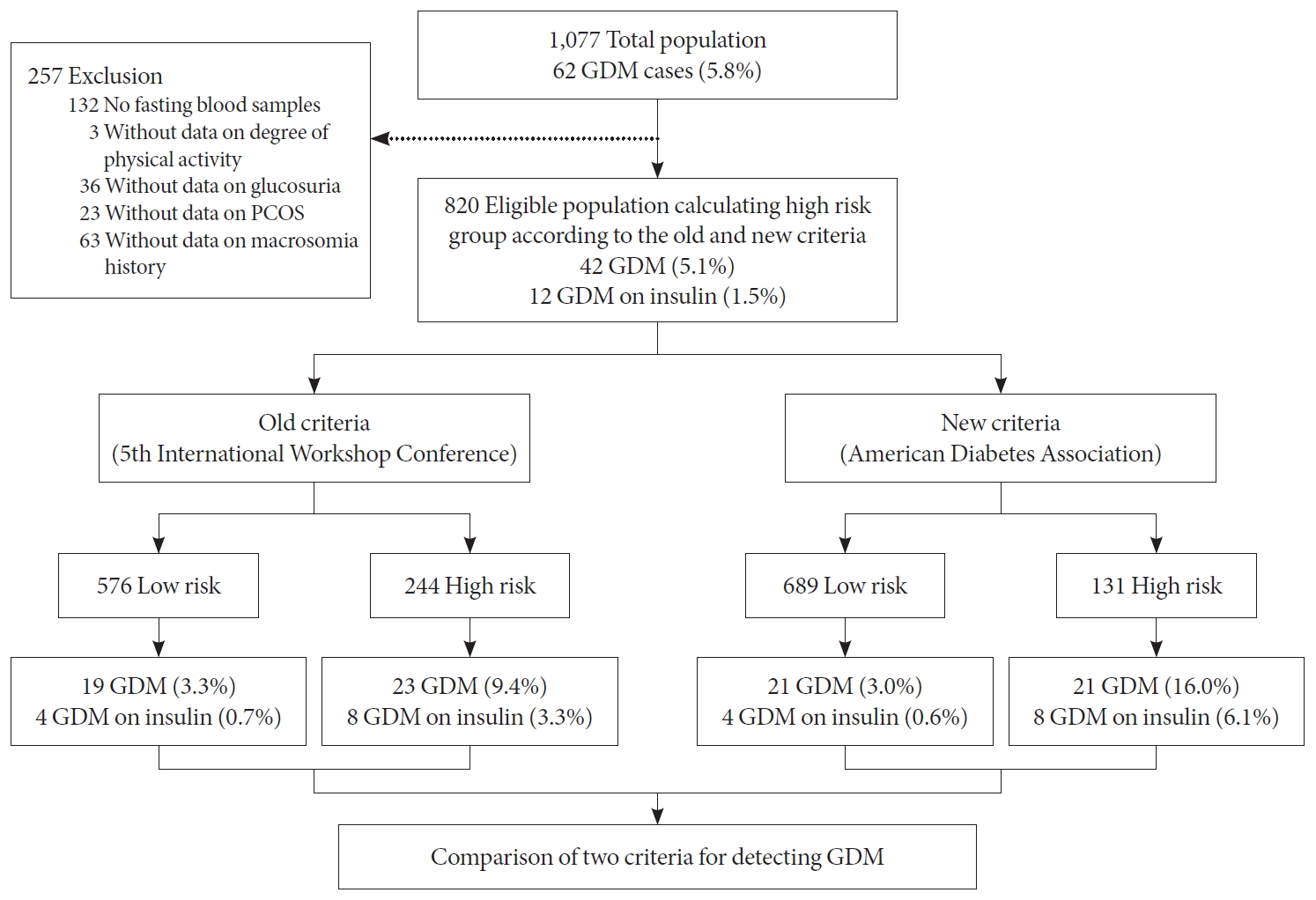
 PubReader
PubReader ePub Link
ePub Link Cite
Cite




