- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 34(5); 2010 > Article
-
ReviewRole of Pyruvate Dehydrogenase Kinase 4 in Regulation of Blood Glucose Levels
- Nam Ho Jeoung1, Robert A. Harris2
-
Korean Diabetes Journal 2010;34(5):274-283.
DOI: https://doi.org/10.4093/kdj.2010.34.5.274
Published online: October 31, 2010
- 5,246 Views
- 67 Download
- 47 Crossref
1Department of Fundamental Medical and Pharmaceutical Sciences, Catholic University of Daegu, Gyeongsan, Korea.
2Department of Biochemistry and Molecular Biology, Indiana University School of Medicine and the Roudebush VA Medical Center, Indianapolis, IN, USA.
- Corresponding author: Robert A. Harris. Department of Biochemistry and Molecular Biology, Indiana University School of Medicine and the Roudebush VA Medical Center, 1481 West 10th Street, Indianapolis, IN 46202, USA. raharris@iupui.edu
Copyright © 2010 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- ABSTRACT
- REGULATION OF PYRUVATE DEHYDROGENASE COMPLEX
- IMPORTANCE OF REGULATION OF PDC BY PDK IN TYPE 2 DIABETES
- ARE THE PDKs VIABLE TARGETS FOR THE TREATMENT OF TYPE 2 DIABETES?
- ROLE OF GLYCERONEOGENESIS IN ACCUMULATION OF FAT IN TISSUES
- KNOCKING OUT PDK4 REDUCES GLUCOSE LEVELS IN FASTING AND STARVATION
- KNOCKING OUT PDK2 HAS NO EFFECT ON BLOOD GLUCOSE LEVELS
- KNOCKING OUT PDK4 LOWERS FASTING BLOOD GLUCOSE LEVELS, IMPROVES GLUCOSE TOLERANCE, AND IMPROVES INSULIN SENSITIVITY IN MICE FED A HIGH UNSATURATED-FAT, HIGH-GLUCOSE DIET
- KNOCKING OUT PDK4 REDUCES ADIPOSITY AND HEPATIC STEATOSIS IN MICE FED A HIGH-SATURATED FAT DIET
- KNOCKING OUT PDK4 INCREASES HEPATIC EXPRESSION OF PGC-1α
- KNOCKING OUT PDK2 TOGETHER WITH PDK4 INDUCES GREATER EFFECTS ON BLOOD GLUCOSE LEVELS, GLUCOSE TOLERANCE, AND INSULIN SENSITIVITY THAN KNOCKING OUT ONLY PDK4
- SUMMARY AND PREDICTIONS
- ACKNOWLEDGMENTS
- REFERENCES
ABSTRACT
- In the well-fed state a relatively high activity of the pyruvate dehydrogenase complex (PDC) reduces blood glucose levels by directing the carbon of pyruvate into the citric acid cycle. In the fasted state a relatively low activity of the PDC helps maintain blood glucose levels by conserving pyruvate and other three carbon compounds for gluconeogenesis. The relative activities of the pyruvate dehydrogenase kinases (PDKs) and the opposing pyruvate dehydrogenase phosphatases determine the activity of PDC in the fed and fasted states. Up regulation of PDK4 is largely responsible for inactivation of PDC in the fasted state. PDK4 knockout mice have lower fasting blood glucose levels than wild type mice, proving that up regulation of PDK4 is important for normal glucose homeostasis. In type 2 diabetes, up regulation of PDK4 also inactivates PDC, which promotes gluconeogenesis and thereby contributes to the hyperglycemia characteristic of this disease. When fed a high fat diet, wild type mice develop fasting hyperglycemia but PDK4 knockout mice remain euglycemic, proving that up regulation of PDK4 contributes to hyperglycemia in diabetes. These finding suggest PDK4 inhibitors might prove useful in the treatment of type 2 diabetes.
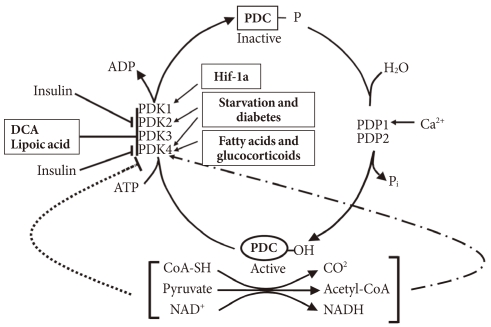
- In the well-fed state the pyruvate dehydrogenase complex (PDC) is converted into its most active dephosphorylated state in order to direct pyruvate into the citric acid cycle. PDC activity is tightly regulated by phosphorylation by four pyruvate dehydrogenase kinases (PDKs) and dephosphorylation by two pyruvate dehydrogenase phosphatases (PDPs) (Fig. 1) [1,2]. The PDKs and PDPs exhibit unique tissue expression patterns, kinetic properties, and sensitivities to regulatory molecules [3,4]. All four PDKs are subject to regulation at the level of transcription. Expression of PDK1 and PDK3 is stimulated by HIF1α [5-7], making these PDKs of considerable interest in hypoxia and cancer. Expression of PDK2 and PDK4 is controlled by nutritional factors and hormones [8-16], making their roles of great interest in starvation and diabetes. PDK3 has received less attention, but is emerging as important since its expression is subject to regulation by HIF1α [7] and the carbohydrate response element binding protein (ChREBP) [17]. Although the PDKs are encoded by genes located in the nucleus, they are markedly different in sequence and three-dimensional structure from other serine protein kinases present in eukaryotic cells, which may facilitate the discovery of specific small molecule inhibitors. Phosphorylation of serine residues of the E1α component of PDC by PDKs inactivates the complex (Fig. 1). Dephosphorylation by PDPs activates the complex. The complex does not exist in any tissue in a completely active, dephosphorylated state nor in a completely inactive, phosphorylated state. The relative activities of the PDKs and the opposing PDPs determine the extent of phosphorylation and therefore the activity of the complex. Induced changes in activities of the kinases relative to the phosphatases during transitions to different nutritional and hormonal states result in substantial change in the phosphorylation state and therefore PDC activity. The products of the overall reaction, acetyl-CoA and NADH, indirectly inhibit the complex by activating the PDKs [18]. A high [NADH]/[NAD+] ratio caused by fatty acid oxidation reduces the lipoyl moieties of E2. A high [acetyl-CoA]/[CoA] ratio caused by fatty acid oxidation promotes acetylation of the reduced lipoyl moieties of E2. Binding of PDK to an E2 lipoyl domain in which the lipoyl group is reduced and acetylated results in maximum kinase activity (Fig. 1) [19]. The consequence is greater E1 phosphorylation and less PDC activity. Whereas the products of PDC promote inactivation of the complex, its substrates (pyruvate, NAD+, and CoA) induce activation by inhibition of the kinases (Fig. 1) [20]. Pyruvate is most important since its concentration fluctuates more dramatically in different nutritional states. Since PDK4 is less sensitive to pyruvate inhibition than PDK2, the shift to greater PDK4 expression during fasting and fat feeding decreases the effectiveness of pyruvate as an activator of PDC in these nutritional states [21].
- During fasting PDC is progressively shut down by phosphorylation in most tissues of the body. High serum levels of free fatty acids (FFAs) generated by lipolysis in the adipose tissue promote fatty acid oxidation in most tissues of the body. Reduced insulin levels and elevated levels of serum FFAs and glucocorticoids induce expression of PDK4 in muscle, kidney, liver, and heart, and PDK2 in liver and kidney. Inhibition of the conversion of pyruvate to acetyl-CoA by decreased PDC activity induced by the PDKs in peripheral tissues, especially skeletal muscle, heart, and liver, conserves three carbon compounds (pyruvate, lactate, and alanine) that are used by the liver to make glucose. Inactivation of the PDC in the liver blocks ketone body synthesis from three carbon compounds but allows ketone body synthesis from fatty acids and ketogenic amino acids. Inhibition of ketogenesis from three carbon compounds is critical because no pathway exists for the conversion of ketone bodies into glucose. Conservation of the compounds that can be used to synthesize glucose at the expense of compounds that can not be converted to glucose (fatty acids, acetyl-CoA, and ketone bodies) helps maintain the blood glucose levels required by the brain and red blood cells. Inactivation of the PDC in starvation indirectly conserves body protein because it minimizes the need for gluconeogenesis from gluconeogenic amino acids and prevents complete oxidation of the carbon skeletons of gluconeogenic amino acids. Indeed, survival during long-term starvation depends upon inactivation of PDC. If the complex remained active in the starved state, the three carbon compounds needed for gluconeogenesis would be converted to CO2 in peripheral tissues and to ketone bodies in the liver. Since survival requires maintenance of glucose levels, animals would have to consume their protein stores at a faster rate as a carbon source for the gluconeogenesis. Since pyruvate is an intermediate in the catabolism of several amino acids, much of the carbon coming from protein would be wasted. Thus, control of the PDC plays an important role in determining the fuel used by tissues in different nutritional and hormonal states.
REGULATION OF PYRUVATE DEHYDROGENASE COMPLEX
- Remarkable upregulation of PDK4 occurs in humans with type 2 diabetes [13,22,23], in genetic animal models of type 2 diabetes [24], in animals fed a high fat diet [21], and in humans fed a high fat diet [25]. Type 2 diabetes is usually the result of a combination of insulin resistance and insulin insufficiency. In the insulin resistance state, the pancreas still makes insulin but the amount released is not enough to normalize blood glucose. Would a PDK inhibitor prove useful in the treatment of type 2 diabetes? Perhaps, but it is not certain. Boosting PDC activity in tissues with a PDK inhibitor will lower blood glucose levels which may reduce the toxic effects of hyperglycemia on the beta cells of the pancreas. However, if the prevailing hypothesis for the mechanism responsible for insulin resistance is correct (Fig. 2) [26], greater PDC activity might exacerbate insulin resistance. Elevated levels of FFAs are associated with inflammation and insulin resistance. A defect in mitochondrial fatty acid oxidation may be responsible. The combination of uptake of too much unesterified fatty acid in the face of reduced or defective fatty acid oxidation results in the accumulation of TAG as well as proinflammatory lipids (fatty acyl-CoA, diacylglycerol, and ceramide) that activate the serine/threonine stress kinases (JNK, IKKβ, and PKCθ) that are responsible for inactivation of components of the insulin signaling cascade [27]. PDK4 deficiency increases blood levels of FFAs and inhibits fatty acid oxidation [28], a combination that would be expected to induce insulin resistance by this mechanism. As to how PDK4 deficiency inhibits fatty acid oxidation, greater PDC activity may lead to an increase in malonyl-CoA, inhibitor of carnitine palmitoyltransferase I (CPT1), the rate-limiting enzyme in fatty acid oxidation. Since long chain acyl-CoA esters are converted to carnitine esters by CPT1, inhibition of CPT1 by malonyl-CoA should increase long chain acyl-CoA esters which in turn may increase diacylglycerol and ceramide, resulting in insulin resistance. It is known that inhibition acetyl-CoA carboxylase which produces malonyl-CoA protects against diet-induced insulin resistance [29]. In spite of what on paper seems should happen, PDK4 deficiency partially protects mice against diet-induced insulin resistance [29]. Although this finding doesn't fit with the model giving in Fig. 2, there are other findings that challenge the current view of the mechanism by which fatty acids induce insulin resistance. For example, data obtained with malonyl-CoA decarboxylase knockout mice also do not support this model [30]. Theoretically, elevated levels of malonyl-CoA in the malonyl-CoA decarboxylase knockout mouse should inhibit CPT1 and thereby increase long chain acyl-CoA esters, diacylglycerol, and ceramide, which in turn should induce insulin resistance by activation of the stress kinases. Instead, malonyl-CoA decarboxylase knockout mice are partially protected, like PDK4-/- mice, against the development of insulin resistance. Based on these findings, Koves et al. [30] proposed that high levels of FFAs may induce a mitochondrial overload or stress by exceeding the capacity of the mitochondria for fatty acid oxidation. They further proposed that mitochondrial overload may induce the accumulation of "incomplete products of fatty acid oxidation," e.g., acyl carnitine ester intermediates that function as proinflammatory compounds responsible for activation of the stress kinases.
IMPORTANCE OF REGULATION OF PDC BY PDK IN TYPE 2 DIABETES
- Upregulation of PDK4 in diabetes begs the question of whether PDK4 and the other PDKs should be considered therapeutic targets for the treatment of diabetes. PDK4-/- mice, generated in an attempt to answer this question, have lower than normal fasting blood glucose levels and slightly but significantly better glucose tolerance [28]. This is observed in both chow-fed PDK4-/- mice that have normal insulin sensitivity [28] and diet-induced obese PDK4-/- mice that are insulin resistant [31]. Dichloroacetate, a well-established PDK inhibitor, decreases fasting blood glucose levels but has relatively low potency and long term treatment causes peripheral neuropathy [32-34]. 2-chloroproprionate also inhibits the PDKs, lowers fasting blood glucose levels, but also induces peripheral neuropathy [35]. α-Lipoic acid also inhibits the PDKs [36], stimulates pyruvate oxidation and inhibits fatty acid oxidation and gluconeogenesis by hepatocytes [37]. These effects likely explain why lipoic acid increases insulin-stimulated glucose disposal in patients with type 2 diabetes [38] and decreases blood levels of lactate and pyruvate [39]. Nevertheless, the mechanism of action of lipoic acid is complicated by its conversion to a CoA ester which inhibits mitochondrial processes by sequestering CoA in the mitochondrial matrix space [40]. The latter inhibits gluconeogenesis [41] and may increase the concentration of AMP and thereby stimulate AMP-activated protein kinase [41]. A synthetic inhibitor of the PDKs, SDZ048-619, increases PDC activity in tissues of the hyperglycemic Zucker diabetic rat and reduces blood lactate but, surprisingly, does not lower blood glucose [42]. In contrast, AZD7545, originally reported to be a specific inhibitor of PDK2 [43], but which we now know also inhibits PDK1 and PDK3 [44], markedly lowers blood glucose in hyperglycemic Zucker diabetic fatty rats [45]. Leelamine, another synthetic inhibitor of the PDKs [46] looks particularly promising as a lead compound. It lowers blood glucose in ob/ob mice [46], and inhibits glyceroneogenesis in isolated adipocytes by activating PDC [47].
ARE THE PDKs VIABLE TARGETS FOR THE TREATMENT OF TYPE 2 DIABETES?
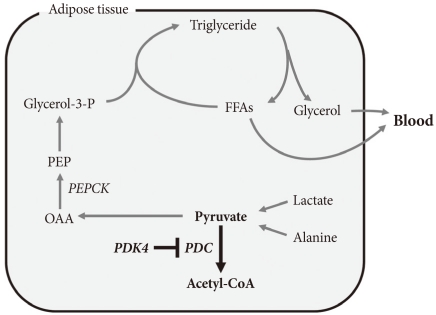
- The term glyceroneogenesis refers to a pathway for the synthesis of the glycerol moiety of TAG from precursors other than glucose (Fig. 3) [48]. The pathway of glyceroneogenesis from lactate, pyruvate, and alanine shares many steps with gluconeogenesis from these substrates. Indeed, like gluconeogenesis, glyceroneogenesis is of greater importance in the fasted state than in the well-fed state. Dietary fat, absorbed as chylomicrons, is cleared from the circulation by adipose tissue lipoprotein lipase which releases FFAs. FFAs are esterified with glycerol 3-phosphate derived from glycolysis (using glucose taken up via insulin-stimulated GLUT4). During fasting, low insulin levels permit the activation of TAG lipases by protein kinase A, hydrolysis of stored TAG, and release of FFAs and glycerol. Much of this FFA is recycled within adipose tissue by re-esterification with glycerol 3-phosphate. Since insulin levels are low, glucose is not available for synthesis of glycerol 3-phosphate. Glycerol 3-phosphate therefore must be generated either by "glyceroneogenesis" from pyruvate, lactate, or alanine, or by phosphorylation of glycerol by glycerol kinase. Glyceroneogenesis is quantitatively much more important than the former [48]. Indeed, like gluconeogenesis, glyceroneogenesis depends on the activity of cytosolic phosphenolpyruvate carboxykinase (PEPCK), which converts oxaloacetate to phosphoenolpyruvate (PEP). Knocking out PEPCK expression in white adipose tissue abolishes glyceroneogenesis from pyruvate [49], resulting in lipodystrophic mice. Conversely, over expression of PEPCK in white adipose tissue increases the rate of glyceroneogenesis from pyruvate, resulting in obese mice [50]. Likewise, over-expression of PEPCK in skeletal muscle results in massive fat accumulation in muscles [51]. Thus, the pathway of glyceroneogenesis plays an important role in intracellular fat accumulation. Although not directly on the pathway of glyceroneogenesis, PDC is positioned to interfere with glyceroneogenesis by the same mechanism that it interferes with gluconeogenesis, i.e., by reducing the availability of lactate, pyruvate, and alanine (Fig. 3). Furthermore, glyceroneogenesis has also been shown to be the most important source of glycerol 3-phosphate for the synthesis of VLDL in the human liver [52]. It is estimated that glucose via glycolysis provides ~15% of glycerol 3-phosphate required for TAG synthesis whereas direct phosphorylation of free glycerol provides ~35% and glyceroneogenesis provides ~50%. Therefore, our working hypothesis is that abolishment of PDK activity can reduce adiposity (and therefore body weight) by limiting the availability of lactate, pyruvate, and alanine for glyceroneogenesis in adipose, liver, and other tissues. Indeed, activation of PDC by inhibition of PDK inhibits glyceroneogenesis in isolated adipocytes [47].
ROLE OF GLYCERONEOGENESIS IN ACCUMULATION OF FAT IN TISSUES
- Strong upregulation of PDK4 during fasting and starvation suggests a dominant role for this PDK in regulation of fuel homeostasis in these conditions. Studies with PDK4-/- mice support this conclusion [28,31,53]. Blood glucose levels are invariably lower in overnight-fasted PDK4-/- mice than overnight-fasted wild type mice. Serum concentrations of lactate, pyruvate, and alanine are also always lower in PDK4-/- mice, consistent with either a faster rate of oxidation by PDC or a reduced rate of production of these compounds. In contrast, higher serum concentrations of FFAs, acetoacetate, β-hydroxybutyrate, and TAG are induced by starvation in PDK4-/- mice relative to wild-type mice. Branched-chain amino acids (BCAAs) are also higher in the blood in starved PDK4-/- mice, consistent with lower blood alanine levels and the importance of BCAAs as a source of amino groups for alanine formation.
- Liver glycogen levels are the same in wild-type and PDK4-/- mice in the fed state but are lost more rapidly from the liver of PDK4-/- mice during fasting. Concentrations of glucose and intermediates of the gluconeogenesis pathway are likewise lower (glucose 6-phosphate, fructose 1,6-bisphosphate, pyruvate, lactate, and citrate) or not different (dihydroxyacetone phosphate, glyceraldehyde 3-phosphate, phosphoenolpyruvate) compared to that of wild-type mice. Ketone bodies are elevated in concentration but with a decrease in the β-hydroxybutyrate to acetoacetate ratio.
- Glucose oxidation rate, measured by 14CO2 production from [U-14C]glucose, was greater in diaphragms from PDK4-/- mice relative to diaphragms from wild-type mice. Rates of lactate release and net glycolysis, measured by 3H2O production from [5-3H]glucose, are significantly reduced. Rates of fatty acid oxidation, measured either by 14CO2 production or acid-soluble product formation from [14C]-Palmitate, are lower in diaphragms obtained from PDK4-/- mice than in diaphragms from wild-type mice. Starvation markedly decreases actual PDC activities in wild-type mice without significantly affecting total PDC activity, thereby decreasing the activity states of PDC to values typical of those induced in tissues of starved rats. A significantly higher PDC activity state is observed in tissues of PDK4-/- mice, consistent with a major role for PDK4 in control of the activity of PDC.
KNOCKING OUT PDK4 REDUCES GLUCOSE LEVELS IN FASTING AND STARVATION
- PDK2-/- mice have also been produced to examine the physiological role of PDK2 in glucose homeostasis (N. H. Jeoung and R. A. Harris, unpublished). They are viable with normal growth characteristics on mouse chow diet. Surprisingly, blood glucose levels are not different from wild-type mice in the fasted state. No difference in glucose levels are observed during the glucose tolerance test relative to age matched wild-type mice. Insulin sensitivity test likewise reveals no difference. PDK2-/- mice maintain normal PDC activities in major tissues and normal blood glucose levels during fasting, perhaps because upregulation of PDK4 compensates for lack of PDK2.
KNOCKING OUT PDK2 HAS NO EFFECT ON BLOOD GLUCOSE LEVELS
- Experiments to determine the effects of knocking out PDK4 on diabetes were carried out with mice fed a high-unsaturated fat, high sucrose diet that is known to induce hyperglycemia and insulin resistance [31]. No differences in blood metabolites were found wild-type and PDK4-/- mice maintained on this diet were compared in the fed state. Significant differences emerged, however, when blood obtained from overnight fasted mice was analyzed [31]. Fasting blood glucose levels were significantly lower in the PDK4-/- mice throughout an 18-week feeding period. PDK4-/- mice were significantly more glucose tolerant than wild-type mice after 16 weeks on the high-fat diet. Insulin levels increased slightly in both groups during the test without significant differences. Nevertheless, insulin tolerance tests revealed significantly greater insulin sensitivity in the PDK4-/- mice after 17 weeks on the diet. Glucose levels were 20% lower in the blood of PDK4-/- mice at the time of their sacrifice after 18 weeks on the high-fat diet. Gluconeogenic precursors (lactate, pyruvate, and alanine) were lower in the PDK4-/- mice but FFAs, TAGs, 3-hydroxybutyrate, acetoacetate, and BCAAs were higher.
- Total PDC activities (completely dephosphorylated state) did not differ between PDK4-/- mice and wild-type mice regardless of whether fed or fasted. The activity states of PDC were significantly higher in the fed state in skeletal muscle and diaphragm of PDK4-/- mice. Relative to the fed state, the activity state of PDC was reduced by overnight fasting in all tissues of PDK4-/- mice with the exception of the kidney. Likewise, in the fasted state the activity state of PDC was significantly higher in the tissues of PDK4-/- mice.
- Diaphragms from PDK4-/- mice oxidize glucose at a rate greater than diaphragms from wild-type mice. Less pyruvate accumulates in the incubation medium with diaphragms from PDK4-/- mice. Palmitate inhibited glucose oxidation in diaphragms from wild-type mice but exerts no inhibition with diaphragms from PDK4-/- mice, indicating inhibition of glucose oxidation by palmitate requires induction of PDK4. The rate of palmitate oxidation is lower in diaphragms isolated from PDK4-/- mice relative to diaphragms from wild-type mice.
KNOCKING OUT PDK4 LOWERS FASTING BLOOD GLUCOSE LEVELS, IMPROVES GLUCOSE TOLERANCE, AND IMPROVES INSULIN SENSITIVITY IN MICE FED A HIGH UNSATURATED-FAT, HIGH-GLUCOSE DIET
- The experiment described above with high unsaturated-fat, high-sucrose fed mice was followed by studies in which wild-type and PDK4-/- mice were fed a high-saturated fat diet (no sucrose) [53] that induce high blood glucose levels, insulin resistance, and (in contrast to high unsaturated-fat, high-sucrose diet) hepatic steatosis [54]. Wild-type and PDK4-/- mice were maintained on this diet for 28 weeks. Fasting blood glucose levels were lower, glucose tolerance improved, and insulin sensitivity greater in the PDK4-/- mice [53]. The activity state (% active) of the PDC was significantly greater in liver and skeletal muscle of PDK4-/- mice after overnight fasting. Serum FFAs and ketone bodies were elevated more in PDK4-/- mice, consistent with slower rates of oxidation. Interestingly, PDK4-/- mice gained less weight than wild-type mice on the diet. Although the two types of mice gained similar amounts of weight for the first 8 weeks on the diet, the wild-type mice gained weight at a faster rate after this point, even though no difference in food consumption could be detected between the two groups. Furthermore, adiposity, as measured by weight of the adipose tissue relative to the body weight, was found to be 13% less in PDK4-/- mice after 28 weeks on the diet. In addition, macrovesicular accumulation of fat was apparent in the livers of wild-type mice by oil red O staining. Livers of PDK4-/- mice also exhibited hepatic steatosis but the fat was microvesicular and less than the amount present in the liver of wild-type mice [53].
KNOCKING OUT PDK4 REDUCES ADIPOSITY AND HEPATIC STEATOSIS IN MICE FED A HIGH-SATURATED FAT DIET
- Hepatic steatosis occurs when the amount of fat delivered to the liver or synthesized de novo exceeds the capacity of the liver to oxidize or secrete. Inhibition of fatty acid oxidation by a mitochondrial poison induces hepatic steatosis. Decreased expression of nuclear-encoded mitochondrial genes is believed responsible for lipid accumulation in skeletal muscle in type 2 diabetes. Reduced mitochondrial capacity for fatty acid oxidation may also be responsible for hepatic steatosis in rodents fed a high saturated-fat diet [55]. PGC-1α, transcription coactivator for several nuclear transcription factors, is a key regulator of mitochondrial biogenesis [56]. Disruption of the PGC-1α gene reduces expression of mitochondrial enzymes, reduces the number of mitochondria, and induces hepatic steatosis [57]. Conversely, activation of PGC-1α by over expression of SIRT1 protects the liver from the accumulation of fat in mice fed a high fat diet. Furthermore, hepatic levels of PGC-1α are reduced in mice fed a high saturated fat diet relative to mice fed a polyunsaturated fat diet [58]. Because an inverse correlation between PGC-1α and tissue fat has been shown in other studies, we determined the amount of PGC-1α present in the livers of wild-type and PDK4-/- mice [53]. As reported previously for liver and muscle of mice fed high saturated-fat diets [58,59], PGC-1α protein was reduced in the liver of wild-type mice fed the high saturated-fat diet compared to wild-type mice fed chow diet. In contrast, a normal amount of PGC-1α was present in the liver of PDK4-/- mice fed the high saturated-fat diet [53]. Since PGC-1α controls expression of mitochondrial enzymes [56,57], these findings suggest that knocking out PDK4 creates conditions that up regulate expression of PGC-1α and therefore genes that encode mitochondrial enzymes in the liver. This is a novel finding that may help explain why knocking out PDK4 reduces fat accumulation in the liver of mice fed a high saturated-fat diet.
KNOCKING OUT PDK4 INCREASES HEPATIC EXPRESSION OF PGC-1α
- PDK2/PDK4 double knockout (DKO) mice are viable and appear normal (N. H. Jeoung and R. A. Harris, unpublished studies). Blood glucose levels of the PDK2/PDK4 DKO mice are lower than that of wild-type mice in both the fed and the fasted state. Overnight fasting induces lower blood levels of lactate, alanine, and pyruvate but higher blood levels of 3-hydroxybutyrate and acetoacetate. Glucose tolerance is remarkably better in the PDK2/PDK4 DKO mice. Insulin levels remain lower during the glucose tolerance test, suggesting the PDK2/PDK4 DKO mice are more sensitive to insulin.
KNOCKING OUT PDK2 TOGETHER WITH PDK4 INDUCES GREATER EFFECTS ON BLOOD GLUCOSE LEVELS, GLUCOSE TOLERANCE, AND INSULIN SENSITIVITY THAN KNOCKING OUT ONLY PDK4
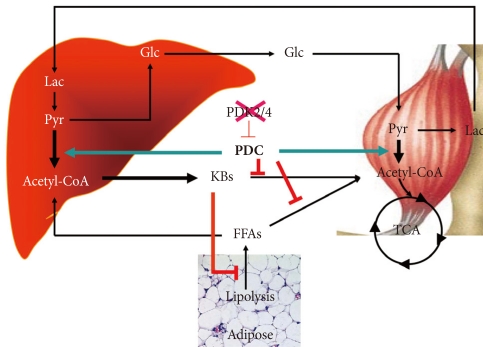
- The schematic given in Fig. 4 summarizes how we visualize knocking out PDK4 affects blood glucose, FFAs, and ketone body levels. Less PDK activity results in greater PDC activity which reduces the levels of lactate, pyruvate, and alanine which attenuates hepatic glucose synthesis and results in lower blood glucose levels. Greater PDC activity results in production of excess acetyl-CoA which the liver converts to ketone bodies. Blood ketone bodies also increase because the increased oxidation of pyruvate inhibits ketone body oxidation by peripheral tissues. It is likely that FFA oxidation is also inhibited in peripheral tissues, but FFAs levels are not greatly elevated, perhaps because lipolysis in adipose tissue is inhibited by the elevated concentrations of ketone bodies [60]. Although complicated, insulin sensitivity may be increased because low PDC activity opposes the action of insulin. Greater PDC activity resulting from reduced PDK activity may facilitate the action of insulin.
- Knocking out PDK4 results in lower blood glucose levels, better glucose tolerance, and greater insulin sensitivity in chow fed mice. The same effects along with reduced hepatic steatosis are observed in PDK4-/- mice fed a high saturated-fat diet. Knocking out both PDK2 and PDK4 lowers blood glucose more effectively and produces even better glucose tolerance and insulin sensitivity in chow fed mice. Based on these findings, we predict that knocking out both PDK2 and PDK4 will have dramatic positive effects on blood glucose, glucose tolerance, and hepatic steatosis in mice fed a high saturated-fat diet. We also anticipate finding greater expression of PGC-1α, more mitochondria, and reduced levels of inflammatory markers in the liver of PDK2/PDK4 DKO mice. Such findings would provide further support for our contention that the PDKs may be good therapeutic targets for the treatment of type 2 diabetes.
SUMMARY AND PREDICTIONS
-
Acknowledgements
- This study was supported by funding from the WCU program through the National Research Foundation of Korea (N. H. J., R. A. H.) and a Merit Award from the Department of Veterans Affairs (R. A. H.) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0008815) (N. H. J.)
ACKNOWLEDGMENTS
- 1. Harris RA, Popov KM, Zhao Y, Kedishvili NY, Shimomura Y, Crabb DW. A new family of protein kinases: the mitochondrial protein kinases. Adv Enzyme Regul 1995;35:147-162. ArticlePubMed
- 2. Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans 2006;34(Pt 2):217-222. ArticlePubMed
- 3. Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J 1998;329(Pt 1):191-196. ArticlePubMedPMCPDF
- 4. Huang B, Gudi R, Wu P, Harris RA, Hamilton J, Popov KM. Isoenzymes of pyruvate dehydrogenase phosphatase. DNA-derived amino acid sequences, expression, and regulation. J Biol Chem 1998;273:17680-17688. PubMed
- 5. Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 2006;3:187-197. ArticlePubMed
- 6. Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 2006;3:177-185. ArticlePubMed
- 7. Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem 2008;283:28106-28114. ArticlePubMedPMC
- 8. Wu P, Sato J, Zhao Y, Jaskiewicz J, Popov KM, Harris RA. Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. Biochem J 1998;329(Pt 1):197-201. ArticlePubMedPMCPDF
- 9. Wu P, Inskeep K, Bowker-Kinley MM, Popov KM, Harris RA. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes 1999;48:1593-1599. ArticlePubMedPDF
- 10. Wu P, Blair PV, Sato J, Jaskiewicz J, Popov KM, Harris RA. Starvation increases the amount of pyruvate dehydrogenase kinase in several mammalian tissues. Arch Biochem Biophys 2000;381:1-7. ArticlePubMed
- 11. Sugden MC, Kraus A, Harris RA, Holness MJ. Fibre-type specific modification of the activity and regulation of skeletal muscle pyruvate dehydrogenase kinase (PDK) by prolonged starvation and refeeding is associated with targeted regulation of PDK isoenzyme 4 expression. Biochem J 2000;346(Pt 3):651-657. ArticlePubMedPMCPDF
- 12. Peters SJ, Harris RA, Heigenhauser GJ, Spriet LL. Muscle fiber type comparison of PDH kinase activity and isoform expression in fed and fasted rats. Am J Physiol Regul Integr Comp Physiol 2001;280:R661-R668. ArticlePubMed
- 13. Majer M, Popov KM, Harris RA, Bogardus C, Prochazka M. Insulin downregulates pyruvate dehydrogenase kinase (PDK) mRNA: potential mechanism contributing to increased lipid oxidation in insulin-resistant subjects. Mol Genet Metab 1998;65:181-186. ArticlePubMed
- 14. Huang B, Wu P, Bowker-Kinley MM, Harris RA. Regulation of pyruvate dehydrogenase kinase expression by peroxisome proliferator-activated receptor-alpha ligands, glucocorticoids, and insulin. Diabetes 2002;51:276-283. PubMed
- 15. Abbot EL, McCormack JG, Reynet C, Hassall DG, Buchan KW, Yeaman SJ. Diverging regulation of pyruvate dehydrogenase kinase isoform gene expression in cultured human muscle cells. FEBS J 2005;272:3004-3014. ArticlePubMed
- 16. Sugden MC, Bulmer K, Gibbons GF, Holness MJ. Role of peroxisome proliferator-activated receptor-alpha in the mechanism underlying changes in renal pyruvate dehydrogenase kinase isoform 4 protein expression in starvation and after refeeding. Arch Biochem Biophys 2001;395:246-252. PubMed
- 17. Burgess SC, Iizuka K, Jeoung NH, Harris RA, Kashiwaya Y, Veech RL, Kitazume T, Uyeda K. Carbohydrate-response element-binding protein deletion alters substrate utilization producing an energy-deficient liver. J Biol Chem 2008;283:1670-1678. ArticlePubMed
- 18. Ravindran S, Radke GA, Guest JR, Roche TE. Lipoyl domain-based mechanism for the integrated feedback control of the pyruvate dehydrogenase complex by enhancement of pyruvate dehydrogenase kinase activity. J Biol Chem 1996;271:653-662. ArticlePubMed
- 19. Baker JC, Yan X, Peng T, Kasten S, Roche TE. Marked differences between two isoforms of human pyruvate dehydrogenase kinase. J Biol Chem 2000;275:15773-15781. ArticlePubMed
- 20. Behal RH, Buxton DB, Robertson JG, Olson MS. Regulation of the pyruvate dehydrogenase multienzyme complex. Annu Rev Nutr 1993;13:497-520. ArticlePubMed
- 21. Holness MJ, Kraus A, Harris RA, Sugden MC. Targeted upregulation of pyruvate dehydrogenase kinase (PDK)-4 in slow-twitch skeletal muscle underlies the stable modification of the regulatory characteristics of PDK induced by high-fat feeding. Diabetes 2000;49:775-781. ArticlePubMedPDF
- 22. Rosa G, Di Rocco P, Manco M, Greco AV, Castagneto M, Vidal H, Mingrone G. Reduced PDK4 expression associates with increased insulin sensitivity in postobese patients. Obes Res 2003;11:176-182. ArticlePubMed
- 23. Spriet LL, Tunstall RJ, Watt MJ, Mehan KA, Hargreaves M, Cameron-Smith D. Pyruvate dehydrogenase activation and kinase expression in human skeletal muscle during fasting. J Appl Physiol 2004;96:2082-2087. ArticlePubMed
- 24. Bajotto G, Murakami T, Nagasaki M, Tamura T, Tamura N, Harris RA, Shimomura Y, Sato Y. Downregulation of the skeletal muscle pyruvate dehydrogenase complex in the Otsuka Long-Evans Tokushima Fatty rat both before and after the onset of diabetes mellitus. Life Sci 2004;75:2117-2130. ArticlePubMed
- 25. Chokkalingam K, Jewell K, Norton L, Littlewood J, van Loon LJ, Mansell P, Macdonald IA, Tsintzas K. High-fat/low-carbohydrate diet reduces insulin-stimulated carbohydrate oxidation but stimulates nonoxidative glucose disposal in humans: An important role for skeletal muscle pyruvate dehydrogenase kinase 4. J Clin Endocrinol Metab 2007;92:284-292. ArticlePubMedPDF
- 26. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111-1119. ArticlePubMedPMC
- 27. Watt MJ, Hevener AL. Fluxing the mitochondria to insulin resistance. Cell Metab 2008;7:5-6. ArticlePubMed
- 28. Jeoung NH, Wu P, Joshi MA, Jaskiewicz J, Bock CB, Depaoli-Roach AA, Harris RA. Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation. Biochem J 2006;397:417-425. ArticlePubMedPMCPDF
- 29. Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, Bhanot S, Monia BP, Shulman GI. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest 2006;116:817-824. ArticlePubMedPMC
- 30. Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45-56. ArticlePubMed
- 31. Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am J Physiol Endocrinol Metab 2008;295:E46-E54. ArticlePubMedPMC
- 32. Stacpoole PW. The pharmacology of dichloroacetate. Metabolism 1989;38:1124-1144. ArticlePubMed
- 33. Crabb DW, Yount EA, Harris RA. The metabolic effects of dichloroacetate. Metabolism 1981;30:1024-1039. ArticlePubMed
- 34. Clark AS, Mitch WE, Goodman MN, Fagan JM, Goheer MA, Curnow RT. Dichloroacetate inhibits glycolysis and augments insulin-stimulated glycogen synthesis in rat muscle. J Clin Invest 1987;79:588-594. ArticlePubMedPMC
- 35. Yount EA, Felten SY, O'Connor BL, Peterson RG, Powell RS, Yum MN, Harris RA. Comparison of the metabolic and toxic effects of 2-chloropropionate and dichloroacetate. J Pharmacol Exp Ther 1982;222:501-508. PubMed
- 36. Korotchkina LG, Sidhu S, Patel MS. R-lipoic acid inhibits mammalian pyruvate dehydrogenase kinase. Free Radic Res 2004;38:1083-1092. ArticlePubMed
- 37. Walgren JL, Amani Z, McMillan JM, Locher M, Buse MG. Effect of R(+)alpha-lipoic acid on pyruvate metabolism and fatty acid oxidation in rat hepatocytes. Metabolism 2004;53:165-173. ArticlePubMed
- 38. Jacob S, Ruus P, Hermann R, Tritschler HJ, Maerker E, Renn W, Augustin HJ, Dietze GJ, Rett K. Oral administration of RAC-alpha-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: a placebo-controlled pilot trial. Free Radic Biol Med 1999;27:309-314. PubMed
- 39. Konrad T, Vicini P, Kusterer K, Hoflich A, Assadkhani A, Bohles HJ, Sewell A, Tritschler HJ, Cobelli C, Usadel KH. Alpha-Lipoic acid treatment decreases serum lactate and pyruvate concentrations and improves glucose effectiveness in lean and obese patients with type 2 diabetes. Diabetes Care 1999;22:280-287. ArticlePubMedPDF
- 40. Blumenthal SA. Inhibition of gluconeogenesis in rat liver by lipoic acid. Evidence for more than one site of action. Biochem J 1984;219:773-780. ArticlePubMedPMCPDF
- 41. Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, Han SM, Kim MS, Jo I, Oh GT, Park IS, Youn JH, Park SW, Lee KU, Park JY. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol 2005;25:2488-2494. PubMed
- 42. Bebernitz GR, Aicher TD, Stanton JL, Gao J, Shetty SS, Knorr DC, Strohschein RJ, Tan J, Brand LJ, Liu C, Wang WH, Vinluan CC, Kaplan EL, Dragland CJ, DelGrande D, Islam A, Lozito RJ, Liu X, Maniara WM, Mann WR. Anilides of (R)-trifluoro-2-hydroxy-2-methylpropionic acid as inhibitors of pyruvate dehydrogenase kinase. J Med Chem 2000;43:2248-2257. ArticlePubMed
- 43. Morrell JA, Orme J, Butlin RJ, Roche TE, Mayers RM, Kilgour E. AZD7545 is a selective inhibitor of pyruvate dehydrogenase kinase 2. Biochem Soc Trans 2003;31(Pt 6):1168-1170. ArticlePubMedPDF
- 44. Kato M, Li J, Chuang JL, Chuang DT. Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate, and radicicol. Structure 2007;15:992-1004. ArticlePubMedPMC
- 45. Mayers RM, Leighton B, Kilgour E. PDH kinase inhibitors: a novel therapy for Type II diabetes? Biochem Soc Trans 2005;33(Pt 2):367-370. ArticlePubMedPDF
- 46. Aicher TD, Damon RE, Koletar J, Vinluan CC, Brand LJ, Gao J, Shetty SS, Kaplan EL, Mann WR. Triterpene and diterpene inhibitors of pyruvate dehydrogenase kinase (PDK). Bioorg Med Chem Lett 1999;9:2223-2228. ArticlePubMed
- 47. Cadoudal T, Distel E, Durant S, Fouque F, Blouin JM, Collinet M, Bortoli S, Forest C, Benelli C. Pyruvate dehydrogenase kinase 4: regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue. Diabetes 2008;57:2272-2279. PubMedPMC
- 48. Reshef L, Olswang Y, Cassuto H, Blum B, Croniger CM, Kalhan SC, Tilghman SM, Hanson RW. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem 2003;278:30413-30416. ArticlePubMed
- 49. Olswang Y, Cohen H, Papo O, Cassuto H, Croniger CM, Hakimi P, Tilghman SM, Hanson RW, Reshef L. A mutation in the peroxisome proliferator-activated receptor gamma-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice. Proc Natl Acad Sci U S A 2002;99:625-630. PubMedPMC
- 50. Franckhauser S, Munoz S, Elias I, Ferre T, Bosch F. Adipose overexpression of phosphoenolpyruvate carboxykinase leads to high susceptibility to diet-induced insulin resistance and obesity. Diabetes 2006;55:273-280. ArticlePubMedPDF
- 51. Hakimi P, Yang J, Casadesus G, Massillon D, Tolentino-Silva F, Nye CK, Cabrera ME, Hagen DR, Utter CB, Baghdy Y, Johnson DH, Wilson DL, Kirwan JP, Kalhan SC, Hanson RW. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J Biol Chem 2007;282:32844-32855. ArticlePubMedPMC
- 52. Kalhan SC, Bugianesi E, McCullough AJ, Hanson RW, Kelley DE. Estimates of hepatic glyceroneogenesis in type 2 diabetes mellitus in humans. Metabolism 2008;57:305-312. ArticlePubMedPMC
- 53. Hwang B, Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) deficiency attenuates the long-term negative effects of a high-saturated fat diet. Biochem J 2009;423:243-252. ArticlePubMedPDF
- 54. Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes--related traits in mouse strains susceptible to diet-induced obesity. Diabetes 2003;52:1958-1966. ArticlePubMedPDF
- 55. Vendemiale G, Grattagliano I, Caraceni P, Caraccio G, Domenicali M, Dall'Agata M, Trevisani F, Guerrieri F, Bernardi M, Altomare E. Mitochondrial oxidative injury and energy metabolism alteration in rat fatty liver: effect of the nutritional status. Hepatology 2001;33:808-815. ArticlePubMed
- 56. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999;98:115-124. ArticlePubMed
- 57. Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 2005;3:e101ArticlePubMedPMC
- 58. You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 2005;42:568-577. ArticlePubMedPMC
- 59. Crunkhorn S, Dearie F, Mantzoros C, Gami H, da Silva WS, Espinoza D, Faucette R, Barry K, Bianco AC, Patti ME. Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J Biol Chem 2007;282:15439-15450. PubMed
- 60. Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, Jin L, Liaw C, Chen R, Richman J, Connolly D, Offermanns S, Wright SD, Waters MG. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 2005;280:26649-26652. PubMed
REFERENCES
Fig. 1Regulation of the pyruvate dehydrogenase complex (PDC) and its kinases (PDKs) and phosphatases (PDPs). DCA, dichloroacetate.

Fig. 2Current model for mechanism responsible for insulin resistance. FFA, free fatty acid; DAG, diacylglycerol; MCD, malonyl-CoA decarboxylase; ACC, acetyl-CoA carboxylase; TAG, triacylglycerol.
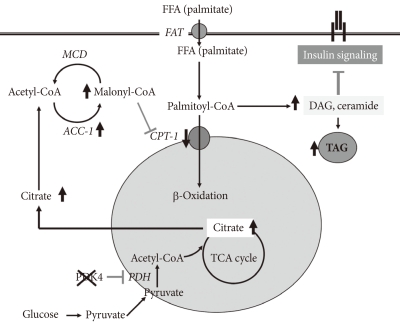

Figure & Data
References
Citations
Citations to this article as recorded by 

- Hepatic fibrosis: Targeting peroxisome proliferator-activated receptor alpha from mechanism to medicines
Lijun Gong, Fang Wei, Frank J. Gonzalez, Guolin Li
Hepatology.2023; 78(5): 1625. CrossRef - microRNA-15b-5p shuttled by mesenchymal stem cell-derived extracellular vesicles protects podocytes from diabetic nephropathy via downregulation of VEGF/PDK4 axis
Tiantian Zhao, Qingsong Jin, Lili Kong, Dongdong Zhang, Yaqin Teng, Liangyan Lin, Xiaoyan Yao, Yongjun Jin, Minglong Li
Journal of Bioenergetics and Biomembranes.2022; 54(1): 17. CrossRef - Circulating exosome-derived miR-122-5p is a novel biomarker for prediction of postoperative atrial fibrillation
Chen Bai, Yisi Liu, Yichen Zhao, Qing Ye, Cheng Zhao, Yang Liu, Jiangang Wang
Journal of Cardiovascular Translational Research.2022; 15(6): 1393. CrossRef - The impact of different feeds on DNA methylation, glycolysis/gluconeogenesis signaling pathway, and gene expression of sheep muscle
Feng Song, Zaccheaus Pazamilala Akonyani, Ying Li, Deqiqige Su, Lantuya Wu, Yue Pang, Sile Hu, Dubala Wu, Chun Li, Ding Yang, Jianghong Wu
PeerJ.2022; 10: e13455. CrossRef - Assessment of hepatic pyruvate carboxylase activity using hyperpolarized [1‐13C]‐l‐lactate
Jun Chen, Edward P. Hackett, Zoltan Kovacs, Craig R. Malloy, Jae Mo Park
Magnetic Resonance in Medicine.2021; 85(3): 1175. CrossRef - Loss of metabolic flexibility as a result of overexpression of pyruvate dehydrogenase kinases in muscle, liver and the immune system: Therapeutic targets in metabolic diseases
Jae‐Han Jeon, Themis Thoudam, Eun Jung Choi, Min‐Ji Kim, Robert A Harris, In‐Kyu Lee
Journal of Diabetes Investigation.2021; 12(1): 21. CrossRef - Exploring the Genetic Conception of Obesity via the Dual Role of FoxO
Tapan Behl, Ishnoor Kaur, Aayush Sehgal, Sukhbir Singh, Gokhan Zengin, Nicoleta Negrut, Delia Carmen Nistor-Cseppento, Flavia Maria Pavel, Raluca Anca Corb Aron, Simona Bungau
International Journal of Molecular Sciences.2021; 22(6): 3179. CrossRef - Pyruvate dehydrogenase kinases (PDKs): an overview toward clinical applications
Xiuxiu Wang, Xiaoyue Shen, Yuting Yan, Hongmin Li
Bioscience Reports.2021;[Epub] CrossRef - PDK2: An Underappreciated Regulator of Liver Metabolism
Benjamin L. Woolbright, Robert A. Harris
Livers.2021; 1(2): 82. CrossRef - Pyruvate dehydrogenase kinase 1 and 2 deficiency reduces high-fat diet-induced hypertrophic obesity and inhibits the differentiation of preadipocytes into mature adipocytes
Hyeon-Ji Kang, Byong-Keol Min, Won-Il Choi, Jae-Han Jeon, Dong Wook Kim, Sungmi Park, Yun-Kyung Lee, Hwa-jin Kim, Ju-Eun Byeon, Younghoon Go, Hye Jin Ham, Yong Hyun Jeon, Mi-Jin Kim, Jung Yi Lee, Adam R. Wende, Sung Hee Choi, Robert A. Harris, In-Kyu Lee
Experimental & Molecular Medicine.2021; 53(9): 1390. CrossRef - Drug evaluation based on phosphomimetic PDHA1 reveals the complexity of activity-related cell death in A549 non-small cell lung cancer cells
Ling Jin, Minkyoung Cho, Bo-Sung Kim, Jung Ho Han, Sungmi Park, In-Kyu Lee, Dongryeol Ryu, Jae Ho Kim, Sung-Jin Bae, Ki-Tae Ha
BMB Reports.2021; 54(11): 563. CrossRef - Aberrant PDK4 Promoter Methylation Preceding Hyperglycemia in a Mouse Model
Sulistyo Emantoko Dwi Putra, Stephanie Singajaya, Ferensia Thesman, Dicky Andhika Pranoto, Ricky Sanjaya, Yoanes Maria Vianney, Ida Bagus Made Artadana
Applied Biochemistry and Biotechnology.2020; 190(3): 1023. CrossRef - Plasma Lipid Profile and Cardiac Risk Markers in Diabetic Nephropathy
Nagendra Subba Rammaiah, Praveenkumar Deverbhavi, Kashinath Rattihalli Thirumala
Journal of Evolution of Medical and Dental Sciences.2020; 9(8): 516. CrossRef - The Role of High-Density Lipoproteins in Endothelial Cell Metabolism and Diabetes-Impaired Angiogenesis
Khalia R. Primer, Peter J. Psaltis, Joanne T.M. Tan, Christina A. Bursill
International Journal of Molecular Sciences.2020; 21(10): 3633. CrossRef - The lncRNA ENST00000608794 acts as a competing endogenous RNA to regulate PDK4 expression by sponging miR-15b-5p in dexamethasone induced steatosis
Fengqiong Liu, Qing Chen, Fa Chen, Jing Wang, Ruijie Gong, Baochang He
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2019; 1864(10): 1449. CrossRef - The Changing Landscape of Pharmacotherapy for Diabetes Mellitus: A Review of Cardiovascular Outcomes
Wu, Gunton
International Journal of Molecular Sciences.2019; 20(23): 5853. CrossRef - Emerging role of the orphan nuclear receptor estrogen-related receptor gamma in liver metabolic diseases
Don-Kyu Kim, Hueng-Sik Choi
Liver Research.2019; 3(2): 99. CrossRef - Characterization of in vitro and in vivo metabolism of leelamine using liquid chromatography-tandem mass spectrometry
Riya Shrestha, Jung Jae Jo, DooHyun Lee, Taeho Lee, Sangkyu Lee
Xenobiotica.2019; 49(5): 577. CrossRef - Proteomics of Rat Lungs Infected by Cryptococcus gattii Reveals a Potential Warburg-like Effect
Rafael L. Rosa, Markus Berger, Lucélia Santi, David Driemeier, Paula Barros Terraciano, Alexandre R. Campos, Jorge A. Guimarães, Marilene H. Vainstein, John R. Yates, Walter O. Beys-da-Silva
Journal of Proteome Research.2019; 18(11): 3885. CrossRef - Dihydrolipoamide dehydrogenase suppression induces human tau phosphorylation by increasing whole body glucose levels in a C. elegans model of Alzheimer’s Disease
Waqar Ahmad
Experimental Brain Research.2018; 236(11): 2857. CrossRef - Peroxisome proliferator-activated receptor β/δ does not regulate glucose uptake and lactose synthesis in bovine mammary epithelial cells cultivated in vitro
Jayant Lohakare, Johan S Osorio, Massimo Bionaz
Journal of Dairy Research.2018; 85(3): 295. CrossRef - Protective potential of Averrhoa bilimbi fruits in ameliorating the hepatic key enzymes in streptozotocin-induced diabetic rats
Surya B Kurup, Mini S
Biomedicine & Pharmacotherapy.2017; 85: 725. CrossRef - Pyruvate dehydrogenase kinase 4 deficiency attenuates cisplatin-induced acute kidney injury
Chang Joo Oh, Chae-Myeong Ha, Young-Keun Choi, Sungmi Park, Mi Sun Choe, Nam Ho Jeoung, Yang Hoon Huh, Hyo-Jeong Kim, Hee-Seok Kweon, Ji-min Lee, Sun Joo Lee, Jae-Han Jeon, Robert A. Harris, Keun-Gyu Park, In-Kyu Lee
Kidney International.2017; 91(4): 880. CrossRef - PDK4 Deficiency Induces Intrinsic Apoptosis in Response to Starvation in Fibroblasts from Doberman Pinschers with Dilated Cardiomyopathy
Kathryn Taggart, Amara Estrada, Patrick Thompson, Francisco Lourenco, Sara Kirmani, Silveli Suzuki-Hatano, Christina A. Pacak
BioResearch Open Access.2017; 6(1): 182. CrossRef - High-salt intake negatively regulates fat deposition in mouse
Huanxian Cui, Shuyan Yang, Maiqing Zheng, Ranran Liu, Guiping Zhao, Jie Wen
Scientific Reports.2017;[Epub] CrossRef - The Effects of Myo-Inositol and B and D Vitamin Supplementation in the db/+ Mouse Model of Gestational Diabetes Mellitus
Jasmine Plows, Florence Budin, Rebecka Andersson, Valerie Mills, Katherine Mace, Sandra Davidge, Mark Vickers, Philip Baker, Irma Silva-Zolezzi, Joanna Stanley
Nutrients.2017; 9(2): 141. CrossRef - Transcriptome analysis illuminates the nature of the intracellular interaction in a vertebrate-algal symbiosis
John A Burns, Huanjia Zhang, Elizabeth Hill, Eunsoo Kim, Ryan Kerney
eLife.2017;[Epub] CrossRef - Equine performance genes and the future of doping in horseracing
Tessa Wilkin, Anna Baoutina, Natasha Hamilton
Drug Testing and Analysis.2017; 9(9): 1456. CrossRef - Urine metabonomic study for blood-replenishing mechanism of Angelica sinensis in a blood-deficient mouse model
Tao WANG, Hong-Guo SUN, Yong-Li HUA, Peng-Ling LI, Yan-Ming WEI
Chinese Journal of Natural Medicines.2016; 14(3): 210. CrossRef - MiR-155 Enhances Insulin Sensitivity by Coordinated Regulation of Multiple Genes in Mice
Xiaolin Lin, Yujuan Qin, Junshuang Jia, Taoyan Lin, Xia Lin, Li Chen, Hui Zeng, Yanjiang Han, Lihong Wu, Shun Huang, Meng Wang, Shenhao Huang, Raoying Xie, Liqi Liang, Yu Liu, Ruiyu Liu, Tingting Zhang, Jing Li, Shengchun Wang, Penghui Sun, Wenhua Huang,
PLOS Genetics.2016; 12(10): e1006308. CrossRef - Antidiabetic efficacy of citronellol, a citrus monoterpene by ameliorating the hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats
Subramani Srinivasan, Udaiyar Muruganathan
Chemico-Biological Interactions.2016; 250: 38. CrossRef - Inflammation increases pyruvate dehydrogenase kinase 4 (PDK4) expression via the Jun N-Terminal Kinase (JNK) pathway in C2C12 cells
Hana Park, Nam Ho Jeoung
Biochemical and Biophysical Research Communications.2016; 469(4): 1049. CrossRef - Whole Blood Gene Expression Differentiates between Atrial Fibrillation and Sinus Rhythm after Cardioversion
Kripa Raman, Stefanie Aeschbacher, Matthias Bossard, Thomas Hochgruber, Andreas J. Zimmermann, Beat A. Kaufmann, Katrin Pumpol, Peter Rickenbacker, Guillaume Paré, David Conen, Alena Talkachova
PLOS ONE.2016; 11(6): e0157550. CrossRef - Soybean Oil Is More Obesogenic and Diabetogenic than Coconut Oil and Fructose in Mouse: Potential Role for the Liver
Poonamjot Deol, Jane R. Evans, Joseph Dhahbi, Karthikeyani Chellappa, Diana S. Han, Stephen Spindler, Frances M. Sladek, Jonathan Peterson
PLOS ONE.2015; 10(7): e0132672. CrossRef - Type 2 diabetes mellitus
Ralph A. DeFronzo, Ele Ferrannini, Leif Groop, Robert R. Henry, William H. Herman, Jens Juul Holst, Frank B. Hu, C. Ronald Kahn, Itamar Raz, Gerald I. Shulman, Donald C. Simonson, Marcia A. Testa, Ram Weiss
Nature Reviews Disease Primers.2015;[Epub] CrossRef - Pyruvate Dehydrogenase Kinases: Therapeutic Targets for Diabetes and Cancers
Nam Ho Jeoung
Diabetes & Metabolism Journal.2015; 39(3): 188. CrossRef - Differential gene expression pattern in hypothalamus of chickens during fasting-induced metabolic reprogramming: Functions of glucose and lipid metabolism in the feed intake of chickens
Xin-Ling Fang, Xiao-Tong Zhu, Sheng-Feng Chen, Zhi-Qi Zhang, Qing-Jie Zeng, Lin Deng, Jian-Long Peng, Jian-Jian Yu, Li-Na Wang, Song-Bo Wang, Ping Gao, Qing-Yan Jiang, Gang Shu
Poultry Science.2014; 93(11): 2841. CrossRef - The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility
Shuai Zhang, Matthew W Hulver, Ryan P McMillan, Mark A Cline, Elizabeth R Gilbert
Nutrition & Metabolism.2014;[Epub] CrossRef - Proteome-Based Systems Biology Analysis of the Diabetic Mouse Aorta Reveals Major Changes in Fatty Acid Biosynthesis as Potential Hallmark in Diabetes Mellitus–Associated Vascular Disease
Holger Husi, Tom Van Agtmael, William Mullen, Ferdinand H. Bahlmann, Joost P. Schanstra, Antonia Vlahou, Christian Delles, Paul Perco, Harald Mischak
Circulation: Cardiovascular Genetics.2014; 7(2): 161. CrossRef - Fatty acid elongase-5 (Elovl5) regulates hepatic triglyceride catabolism in obese C57BL/6J mice
Sasmita Tripathy, Kelli A. Lytle, Robert D. Stevens, James R. Bain, Christopher B. Newgard, Andrew S. Greenberg, Li-Shin Huang, Donald B. Jump
Journal of Lipid Research.2014; 55(7): 1448. CrossRef - Mechanisms for the adverse effects of late gestational increases in maternal cortisol on the heart revealed by transcriptomic analyses of the fetal septum
Elaine M. Richards, Charles E. Wood, Maria Belen Rabaglino, Andrew Antolic, Maureen Keller-Wood
Physiological Genomics.2014; 46(15): 547. CrossRef - Novel Agents for the Treatment of Type 2 Diabetes
Ralph A. DeFronzo, Curtis L. Triplitt, Muhammad Abdul-Ghani, Eugenio Cersosimo
Diabetes Spectrum.2014; 27(2): 100. CrossRef - Serotonin (5-HT) Affects Expression of Liver Metabolic Enzymes and Mammary Gland Glucose Transporters during the Transition from Pregnancy to Lactation
Jimena Laporta, Tonia L. Peters, Kathryn E. Merriman, Chad M. Vezina, Laura L. Hernandez, Julie A. Chowen
PLoS ONE.2013; 8(2): e57847. CrossRef - Dietary supplementation with vitamin E and C attenuates dexamethasone-induced glucose intolerance in rats
Deon B. Williams, Zhongxiao Wan, Bruce C. Frier, Rhonda C. Bell, Catherine J. Field, David C. Wright
American Journal of Physiology-Regulatory, Integrative and Comparative Physiology.2012; 302(1): R49. CrossRef - Association of pyruvate dehydrogenase kinase 4 gene polymorphisms with type 2 diabetes and metabolic syndrome
Seong-Su Moon, Jung-Eun Lee, Young-Sil Lee, Su-Won Kim, Nam Ho Jeoung, In-Kyu Lee, Jung-Guk Kim
Diabetes Research and Clinical Practice.2012; 95(2): 230. CrossRef - Sex-dependent differences in rat hepatic lipid accumulation and insulin sensitivity in response to diet-induced obesity
Antònia Nadal-Casellas, Ana Maria Proenza, Isabel Lladó, Magdalena Gianotti
Biochemistry and Cell Biology.2012; 90(2): 164. CrossRef - Prednisolone-induced changes in gene-expression profiles in healthy volunteers
Erik JM Toonen, Wilco WM Fleuren, Ulla Nässander, Marie-José C van Lierop, Susanne Bauerschmidt, Wim HA Dokter, Wynand Alkema
Pharmacogenomics.2011; 12(7): 985. CrossRef

 KDA
KDA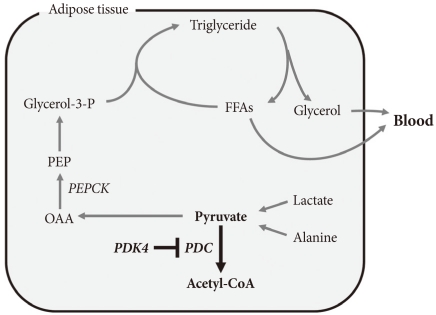
 PubReader
PubReader Cite
Cite