- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 39(6); 2015 > Article
-
ReviewPathophysiology Morphologic Changes in Autonomic Nerves in Diabetic Autonomic Neuropathy
- Heung Yong Jin, Hong Sun Baek, Tae Sun Park
-
Diabetes & Metabolism Journal 2015;39(6):461-467.
DOI: https://doi.org/10.4093/dmj.2015.39.6.461
Published online: December 11, 2015
Division of Endocrinology and Metabolism, Department of Internal Medicine, Research Institute of Clinical Medicine, Chonbuk National University Hospital, Chonbuk National University Medical School, Jeonju, Korea.
- Corresponding author: Tae Sun Park. Division of Endocrinology and Metabolism, Department of Internal Medicine, Research Institute of Clinical Medicine, Chonbuk National University Hospital, Chonbuk National University Medical School, 20 Geonji-ro, Deokjin-gu, Jeonju 54907, Korea. pts@jbnu.ac.kr
• Received: November 4, 2015 • Accepted: November 25, 2015
Copyright © 2015 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Diabetic neuropathy is one of the major complications of diabetes, and it increases morbidity and mortality in patients with both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). Because the autonomic nervous system, for example, parasympathetic axons, has a diffuse and wide distribution, we do not know the morphological changes that occur in autonomic neural control and their exact mechanisms in diabetic patients with diabetic autonomic neuropathy (DAN). Although the prevalence of sympathetic and parasympathetic neuropathy is similar in T1DM versus T2DM patients, sympathetic nerve function correlates with parasympathetic neuropathy only in T1DM patients. The explanation for these discrepancies might be that parasympathetic nerve function was more severely affected among T2DM patients. As parasympathetic nerve damage seems to be more advanced than sympathetic nerve damage, it might be that parasympathetic neuropathy precedes sympathetic neuropathy in T2DM, which was Ewing's concept. This could be explained by the intrinsic morphologic difference. Therefore, the morphological changes in the sympathetic and parasympathetic nerves of involved organs in T1DM and T2DM patients who have DAN should be evaluated. In this review, evaluation methods for morphological changes in the epidermal nerves of skin, and the intrinsic nerves of the stomach will be discussed.
- Diabetic autonomic neuropathy (DAN) is one of the major diabetic complications, and it increases morbidity and mortality in patients with both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) [1]. It can involve sensory, motor, and autonomic nerves and all three types of involvement can coexist. In the past century, however, DAN has been considered to be a rare complication. Currently DAN is considered to be a serious and underestimated frequent complication of diabetes [2]. The first reason is that DAN is a systemic disease that involves every organ in the body by affecting the entire autonomic nervous system (ANS) and it leads to increase in morbidity and mortality. The second reason is that DAN is almost always asymptomatic or reveals non-specific symptoms in the early stages, and, therefore, many clinicians fail to recognize the opportunity for early diagnosis and treatment [3]. DAN has specific clinical abnormalities by hyperglycemia and hypoglycemia in skin, cardiovascular, gastrointestinal, genitourinary, and other organs [3].
- Although some epidemiologic data on diabetic peripheral neuropathy has been known to be existed [45], very limited epidemiologic data for DAN had been published [67]. More than 50% of patients with T2DM had been reported to have DAN [7] and 45.3% of patients with newly detected T2DM had DAN at the time of diagnosis [8]. However, early diagnosis of DAN is very difficult because confirmed diagnostic parameters and criteria do not exist. Therefore, in the absence of exact diagnostic criteria for DAN, objective evaluation and diagnostic methods of DAN are needed. Recently, we commonly used autonomic function test rather than skin and target organ biopsy because of its convenience. However, autonomic function test is indirect test that influenced by the functional status and dual innervations of target organs. Therefore, autonomic function test and morphological study has some differences in the diagnosis of DAN. Compare to autonomic function tests, morphological studies have high sensitivity and specificity for DAN diagnosis and discriminates between the autonomic and somatic nerve, and, also sympathetic and parasympathetic nerve. So, the usefulness of target organ biopsy should be evaluated and introduced for DAN diagnosis.
INTRODUCTION
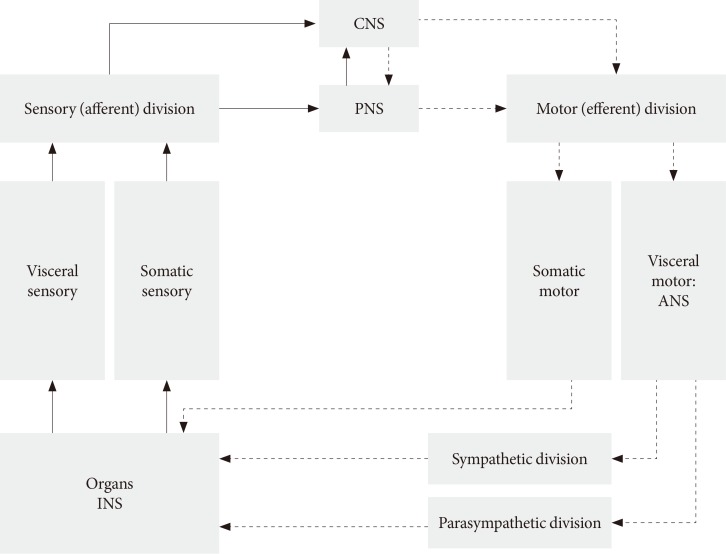
- The ANS usually works without our will and controls many functions of all innervated visceral organs. It operates throughout the body to adapt organ function to changes in the internal and external environments. Afferent sensory division integrates all sensory input in neural centers located mainly in the brainstem and the hypothalamus. It operates, in part, through visceral reflexes in which sensory signals from visceral organs activate central autonomic regions that, in turn, send back subconscious reflex responses to visceral organs to change their activities (Fig. 1). The efferent autonomic signals are transmitted to the organs through the parasympathetic and sympathetic nervous systems [9]. Because the ANS, for example parasympathetic axons, have a diffuse and wide distribution, we do not know the exact mechanism and morphological changes that occur in autonomic neural control in diabetic patients with DAN. DAN includes both parasympathetic and sympathetic nerve dysfunction in both T1DM and T2DM patients. It is unknown what the changes and role of the ANS are during the progression of diabetes?
- The prevalence of sympathetic and parasympathetic neuropathy, respectively, is similar in T1DM versus T2DM patients. Sympathetic nerve function correlates with parasympathetic neuropathy only in T1DM patients [10]. The explanation for these discrepancies might be that parasympathetic nerve function is more severely affected in T2DM patients. As parasympathetic nerve damage seemed to be more advanced than sympathetic nerve damage, it might be that parasympathetic neuropathy precedes sympathetic neuropathy in T2DM, which was like Ewing's concept [1011]. This could be explained by basic morphological difference in autonomic nerves. Parasympathetic nerves are composed of large nerve fibers because they have longer and thicker myelinated preganglionic fibers, sympathetic nerves are thinner and longer nonmyelinated postganglionic fibers. The fact that impaired glucose tolerance patients may develop both parasympathetic and peripheral sensory neuropathy suggests that large myelinated nerve fibers are affected early in T2DM [12]. Therefore, the order of morphological changes in sympathetic and parasympathetic nerves of various organs in diabetic patients must be evaluated.
- Autonomic dysfunction (e.g., heightened activity of the sympathetic nervous system and suppressed activity of the parasympathetic nervous system) impairs the ability of the ANS to regulate the cardiovascular system, and inflammatory, metabolic and neurological disease processes [213]. Autonomic imbalance may be shown to be a key component involved in both the etiology and clinical course of cardiovascular, gastrointestinal, genitourinary, and other organs. Therefore, the role of autonomic imbalance and mechanisms in DAN require further exploration.
AUTONOMIC NERVOUS SYSTEM IN DAN
- The protein gene product (PGP) 9.5 as an ubiquitin hydrolase component of axons [14] provided unequivocal evidence of the presence of unmyelinated nerve fibers in the epidermis of the skin [15], stomach [16], kidney [17], and pancreas [1819]. This method is an important diagnostic tool for small fiber sensory neuropathy and autonomic neuropathy according to qualitative and quantitative studies of sensory and autonomic nerve fiber densities and morphology in diabetic research.
- Skin biopsy is a useful tool to diagnose small fiber sensory neuropathy in clinical practice and to monitor the progression of neuropathy using immunohistochemical techniques. This morphometric analysis method proved to be reliable, reproducible and unaffected by the severity of neuropathy [20]. Recently, a rapid and innovative method, called optical clearing (use of immersion solution to reduce scattering) [21] which was introduced by Fu et al. [19] and Tang et al. [22] can be used to distinguish sensory nerve fibers from autonomic nerve fibers very clearly. In the epidermis, unmyelinated sensory nerve fibers were innervated. These intraepidermal nerve fibers run to the skin surface or are divided with horizontal branches [23]. They can be labeled with antibodies such as, PGP 9.5 and transient receptor potential vanilloid type 1 (TRPV1). The subpapillary dermis of both hairy and glabrous skin is innervated by bundles of unmyelinated and thinly myelinated fibers and glabrous skin also contains large myelinated fibers. In the dermis, autonomic structures such as sweat glands, blood vessels, arrector pili muscles, and hair follicles existed. They could be evaluated by the various antibodies; for example, tyrosine hydroxylase (TH) and dopamine β hydroxylase (DβH) for adrenergic sympathetic fibers (anti-TH, anti-DβH), neuropeptide Y for noradrenergic fibers, vasoactive intestinal peptide (VIP) for cholinergic fibers (anti-VIP), and calcitonin gene related peptide (CGRP) and substance P (SP) for vasodilatory peptidergic fibers (anti-CGRP, anti-SP) (Table 1) [24].
- Morphometric analysis of skin nerves is readily accomplished through the use of immunohistochemical techniques, and has proved to be reliable, reproducible and unaffected by the severity of neuropathy. One further advantage of skin biopsy over conventional nerve biopsy is that it allows somatic nerve fibers to be distinguished from autonomic nerve fibers. Tissue processing methods of skin samples depend on subsequent visualization techniques. For immunohistochemistry, fixation is typically performed with paraformaldehyde. Immunoperoxidase staining allows bright-field quantification, and immunofluorescent staining can be coupled with either epifluorescence microscopy or confocal microscopy. Species-specific secondary antibodies may be used to visualize multiple antigen-bound primary antibodies selected in a number of combinations to investigate neuronal structures, cutaneous structures, and neurotransmitters relevant to the sensory system and the autonomic system (Table 1) [24]. Gibbons et al. [25] performed degeneration and regeneration experiments on cutaneous autonomic nerve fibers by using a topical capsaicin application in healthy subjects. They found that sudomotor, vasomotor, and pilomotor nerve fibers are degenerated and impaired function. The autonomic nerve fibers tend to degenerate more gradually and recover more rapidly than sensory fibers. Therefore, they suggest nerve susceptibility and/or pathophysiological mechanisms of nerve degeneration and regeneration may differ between autonomic and sensory nerve fibers treated with capsaicin [25]. As seen from these results, further studies on the degeneration sequences of autonomic nerves are necessary to define which autonomic nerve function is affected earlier by hyperglycemia and hypoglycemia in humans.
MORPHOLOGICAL CHANGES OF SKIN IN DAN
- Anatomically, the gastrointestinal tract has many neuronal populations, such as, enteric (intrinsic) neurons, extrinsic sensory afferent neurons, viscerofugal neurons, sympathetic neurons, and parasympathetic neurons. Intrinsic neurons have cell bodies and processes within the gut wall. Viscerofugal neurons which the cell bodies located in the myenteric plexus, project to postganglionic sympathetic visceromotor neurons in the prevertebral ganglia along the extrinsic nerve trunk. Postganglionic sympathetic neurons project to the gut wall and form the efferent limb. The other efferent limb of the CNS arises from parasympathetic motor neurons [26]. In the stomach, the gastric submucosa contains few intrinsic primary afferent neurons compare to small intestines.
- We published the morphological comparison of gastric mucosal nerve fibers in T2DM and non-diabetic patients and suggested that the gastric mucosal nerve density can be used as a biomarker of gastric autonomic neuropathy [16]. Selim et al. [27] also suggested that gastric mucosal density can be a practical method for histologic diagnosis of gastric autonomic neuropathy in T1DM patients. The autonomic neuropathic stomach showed decreased mucosal nerve fiber length and volume density, and an altered mucosal nerve fiber innervation pattern in animal diabetes model (Fig. 2) and human diabetics. Although gastric mucosal nerve fiber loss is associated with diabetic sensory peripheral neuropathy and diabetes duration, intraepidermal nerve fiber density could not predict mucosal nerve fiber deficiency. So, intraepidermal nerve fiber density cannot be used as a surrogate marker of gastric mucosal nerve density.
- Morphology and innervation of mucosal and muscular nerve fibers defined by immunoreactivity of neurochemicals including TH, SP, CGRP, and VIP. Using immunohistochemical studies, gastric sensory neuronal axons, parasympathetic and sympathetic axons, endocrine cells and vascular cells localization and distribution can be revealed. The parasympathetic innervation was identified by the presence of cholinergic marker antibodies (e.g., vesicular acetylcholine transporter, choline acetyltransferase, and VIP) and the sympathetic innervation by the expression of catecholaminergic markers antibodies (TH).
- Gastric mucosal neuropeptide immunohistochemical distribution of VIP, CGRP, and SP differed by disease [28] and location [27].
- For example, VIP positive nerve fibers were abundant in the fundus compared to SP positive nerve fibers, and VIP and SP positive nerve fibers were almost the same in the antrum. Both VIP and SP positive nerve fibers were significantly decreased in diabetic patients. Although gastric mucosal nerve fiber length and density evaluation is a useful method in the presence of autonomic neuropathy, we do not know the status of autonomic nerve involvement. Therefore, we need a precise evaluation method for sympathetic and parasympathetic nerve fiber innervation and degeneration.
MORPHOLOGICAL CHANGES IN AUTONOMIC NERVES IN GASTRIC AUTONOMIC NEUROPATHY
- In clinical practice, various autonomic function tests have been proposed to study patients with suspected autonomic neuropathy. DAN involves every organ in the body and shows various symptoms matching each organ. To diagnose specific autonomic neuropathy, we must perform specific tests. The sensitivity and specificity of autonomic function tests vary and a combination of various autonomic tests comprising a composite scoring scale [29] has been suggested to be a maximally sensitive and specific approach. Last year, Thaisetthawatkul et al. [30] suggested that autonomic evaluation is independent of somatic evaluation for small fiber neuropathy. They suggested that autonomic and sensory function tests are independent of small fiber neuropathy. Therefore, both autonomic and sensory tests should be performed concurrently for distal small fiber neuropathy diagnosis. Recently, skin biopsy has been widely used as a diagnostic method for somatic and autonomic neuropathy. Skin biopsy has several advantages for sensory and autonomic neuropathy evaluation because it is a less invasive procedure and can be repeated to monitor disease progression. Additionally, immunohistochemistry with an optical clearing process can discriminate the sympathetic and parasympathetic nerve fibers. In the stomach, endoscopic mucosal biopsy has the same advantages and can be used for histological diagnosis of autonomic neuropathy and optical clearing with immunohistochemistry can observe the sympathetic and parasympathetic nerve innervation and morphologic changes in diabetic patients. Introduction of optic clearing and the development of three-dimensional visualization can be used to observe the skin, stomach, pancreas, and kidney with high resolution. These advances enable the evaluation and monitoring of the autonomic nerve fibers, such as sympathetic and parasympathetic nerve fiber innervation, morphologic changes, and degeneration and regeneration sequences.
CONCLUSIONS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
NOTES
- 1. Pasnoor M, Dimachkie MM, Kluding P, Barohn RJ. Diabetic neuropathy part 1: overview and symmetric phenotypes. Neurol Clin 2013;31:425-445. PubMedPMC
- 2. Vinik AI, Maser RE, Ziegler D. Autonomic imbalance: prophet of doom or scope for hope? Diabet Med 2011;28:643-651. ArticlePubMedPMC
- 3. Verrotti A, Prezioso G, Scattoni R, Chiarelli F. Autonomic neuropathy in diabetes mellitus. Front Endocrinol (Lausanne) 2014;5:205ArticlePubMedPMC
- 4. Kim SS, Won JC, Kwon HS, Kim CH, Lee JH, Park TS, Ko KS, Cha BY. Prevalence and clinical implications of painful diabetic peripheral neuropathy in type 2 diabetes: results from a nationwide hospital-based study of diabetic neuropathy in Korea. Diabetes Res Clin Pract 2014;103:522-529. ArticlePubMed
- 5. Won JC, Kim SS, Ko KS, Cha BY. Current status of diabetic peripheral neuropathy in Korea: report of a hospital-based study of type 2 diabetic patients in Korea by the diabetic neuropathy study group of the Korean Diabetes Association. Diabetes Metab J 2014;38:25-31. ArticlePubMedPMC
- 6. Ko SH, Park SA, Cho JH, Song KH, Yoon KH, Cha BY, Son HY, Yoo KD, Moon KW, Park YM, Ahn YB. Progression of cardiovascular autonomic dysfunction in patients with type 2 diabetes: a 7-year follow-up study. Diabetes Care 2008;31:1832-1836. PubMedPMC
- 7. Ko SH, Song KH, Park SA, Kim SR, Cha BY, Son HY, Moon KW, Yoo KD, Park YM, Cho JH, Yoon KH, Ahn YB. Cardiovascular autonomic dysfunction predicts acute ischaemic stroke in patients with type 2 diabetes mellitus: a 7-year follow-up study. Diabet Med 2008;25:1171-1177. ArticlePubMed
- 8. Koo BK. Screening of autonomic neuropathy in patients with type 2 diabetes. Diabetes Metab J 2014;38:346-348. ArticlePubMedPMC
- 9. Rodriguez-Diaz R, Caicedo A. Neural control of the endocrine pancreas. Best Pract Res Clin Endocrinol Metab 2014;28:745-756. ArticlePubMed
- 10. Freccero C, Svensson H, Bornmyr S, Wollmer P, Sundkvist G. Sympathetic and parasympathetic neuropathy are frequent in both type 1 and type 2 diabetic patients. Diabetes Care 2004;27:2936-2941. ArticlePubMedPDF
- 11. Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology 2001;57:1701-1704. ArticlePubMed
- 12. Wu JS, Yang YC, Lin TS, Huang YH, Chen JJ, Lu FH, Wu CH, Chang CJ. Epidemiological evidence of altered cardiac autonomic function in subjects with impaired glucose tolerance but not isolated impaired fasting glucose. J Clin Endocrinol Metab 2007;92:3885-3889. ArticlePubMed
- 13. Ondicova K, Mravec B. Multilevel interactions between the sympathetic and parasympathetic nervous systems: a minireview. Endocr Regul 2010;44:69-75. ArticlePubMed
- 14. Wang L, Hilliges M, Jernberg T, Wiegleb-Edstrom D, Johansson O. Protein gene product 9.5-immunoreactive nerve fibres and cells in human skin. Cell Tissue Res 1990;261:25-33. ArticlePubMedPDF
- 15. Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes Care 2004;27:1974-1979. ArticlePubMedPDF
- 16. Jin HY, Kang YM, Kim CY, Kim SH, Liu WJ, Piao MH, Park JH, Baek HS, Park TS. Morphological comparison of small nerve fibres in gastric mucosa in non-diabetic and type 2 diabetic subjects. Diabet Med 2009;26:943-946. ArticlePubMed
- 17. Wilson PO, Barber PC, Hamid QA, Power BF, Dhillon AP, Rode J, Day IN, Thompson RJ, Polak JM. The immunolocalization of protein gene product 9.5 using rabbit polyclonal and mouse monoclonal antibodies. Br J Exp Pathol 1988;69:91-104. PubMedPMC
- 18. Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 2011;14:45-54. ArticlePubMedPMC
- 19. Fu YY, Peng SJ, Lin HY, Pasricha PJ, Tang SC. 3-D imaging and illustration of mouse intestinal neurovascular complex. Am J Physiol Gastrointest Liver Physiol 2013;304:G1-G11. ArticlePubMed
- 20. Donadio V, Incensi A, Giannoccaro MP, Cortelli P, Di Stasi V, Pizza F, Jaber MA, Baruzzi A, Liguori R. Peripheral autonomic neuropathy: diagnostic contribution of skin biopsy. J Neuropathol Exp Neurol 2012;71:1000-1008. ArticlePubMed
- 21. Bui AK, McClure RA, Chang J, Stoianovici C, Hirshburg J, Yeh AT, Choi B. Revisiting optical clearing with dimethyl sulfoxide (DMSO). Lasers Surg Med 2009;41:142-148. ArticlePubMedPMC
- 22. Tang SC, Peng SJ, Chien HJ. Imaging of the islet neural network. Diabetes Obes Metab 2014;16(Suppl 1):77-86. ArticlePubMed
- 23. Kennedy WR, Wendelschafer-Crabb G. The innervation of human epidermis. J Neurol Sci 1993;115:184-190. ArticlePubMed
- 24. Myers MI, Peltier AC. Uses of skin biopsy for sensory and autonomic nerve assessment. Curr Neurol Neurosci Rep 2013;13:323ArticlePubMedPMCPDF
- 25. Gibbons CH, Wang N, Freeman R. Capsaicin induces degeneration of cutaneous autonomic nerve fibers. Ann Neurol 2010;68:888-898. ArticlePubMedPMC
- 26. Brierley SM, Linden DR. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol 2014;11:611-627. ArticlePubMedPDF
- 27. Selim MM, Wendelschafer-Crabb G, Redmon JB, Khoruts A, Hodges JS, Koch K, Walk D, Kennedy WR. Gastric mucosal nerve density: a biomarker for diabetic autonomic neuropathy? Neurology 2010;75:973-981. ArticlePubMedPMC
- 28. Domotor A, Peidl Z, Vincze A, Hunyady B, Szolcsanyi J, Kereskay L, Szekeres G, Mozsik G. Immunohistochemical distribution of vanilloid receptor, calcitonin-gene related peptide and substance P in gastrointestinal mucosa of patients with different gastrointestinal disorders. Inflammopharmacology 2005;13:161-177. ArticlePubMed
- 29. Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc 1993;68:748-752. ArticlePubMed
- 30. Thaisetthawatkul P, Fernandes Filho JA, Herrmann DN. Autonomic evaluation is independent of somatic evaluation for small fiber neuropathy. J Neurol Sci 2014;344:51-54. ArticlePubMed
REFERENCES
Fig. 1
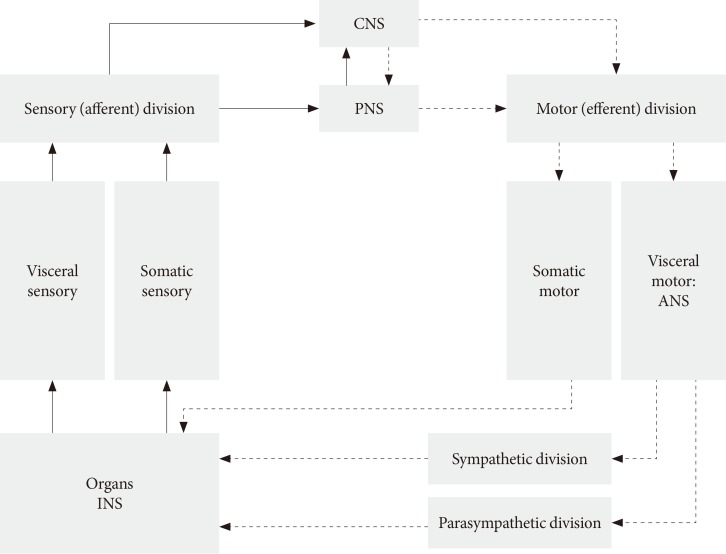
Structure of autonomic nervous system (ANS). CNS, central nervous system; PNS, peripheral nervous system; INS, intrinsic nervous system.

Fig. 2
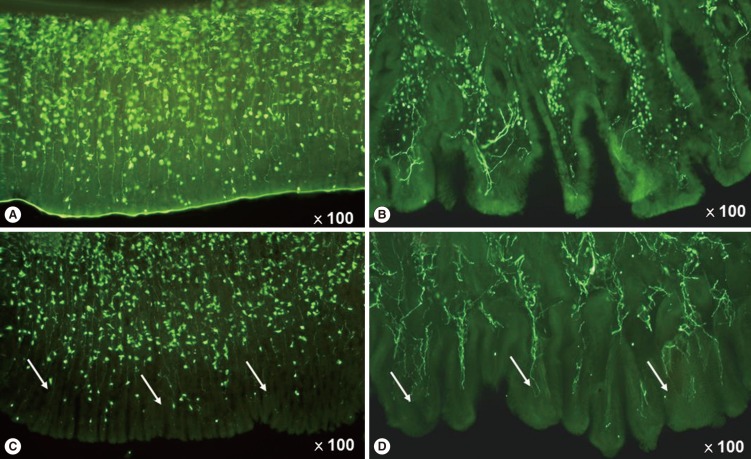
Diabetic rat gastric mucosal layer change in human and animal models by the protein gene product 9.5 positive fibers. (A) rat control, (B) human control, (C) rat diabetes mellitus (DM), and (D) human DM. (C, D) Arrows indicated the gastric mucosal nerve degeneration of rat DM model and human DM.

Table 1
![dmj-39-461-i001.jpg]()
Target and its antibody of immunoreactive skin structures
Adapted from Myers et al. [24], with permission from Springer.
Figure & Data
References
Citations
Citations to this article as recorded by 

- Impaired Cardiovagal Activity as a Link Between Hyperglycemia and Arterial Stiffness in Adults With Type 2 Diabetes Mellitus Patients Among an Eastern Indian Population: A Cross-sectional Study
Nibedita Priyadarsini, Devineni Likhitha, Madumathy Ramachandran, Kishore Kumar Behera
Canadian Journal of Diabetes.2023;[Epub] CrossRef - Diabetic visceral neuropathy of gastroparesis: Gastric mucosal innervation and clinical significance
Ping‐Huei Tseng, Chi‐Chao Chao, Ya‐Yin Cheng, Chieh‐Chang Chen, Ping‐Hao Yang, Wei‐Kang Yang, Shao‐Wei Wu, Yen‐Wen Wu, Mei‐Fang Cheng, Wei‐Shiung Yang, Ming‐Shiang Wu, Sung‐Tsang Hsieh
European Journal of Neurology.2022; 29(7): 2097. CrossRef - Pathogenesis of Distal Symmetrical Polyneuropathy in Diabetes
Sasha Smith, Pasha Normahani, Tristan Lane, David Hohenschurz-Schmidt, Nick Oliver, Alun Huw Davies
Life.2022; 12(7): 1074. CrossRef - Diabetic Cardiomyopathy and Ischemic Heart Disease: Prevention and Therapy by Exercise and Conditioning
Antonio Crisafulli, Pasquale Pagliaro, Silvana Roberto, Lucia Cugusi, Giuseppe Mercuro, Antigone Lazou, Christophe Beauloye, Luc Bertrand, Derek J. Hausenloy, Manuela Aragno, Claudia Penna
International Journal of Molecular Sciences.2020; 21(8): 2896. CrossRef - Distribution characteristics of sweat gland nerve fibres in normal humans identified by acetylcholinesterase histochemical staining
Li Ling, Yongdan Liu, Yifei Sun, Yun Cai, Ye Jiang, Longjian Chen, Long He, Jinwei Xue
Clinical Neurology and Neurosurgery.2020; 189: 105620. CrossRef - Diabetes abolish cardioprotective effects of remote ischemic conditioning: evidences and possible mechanisms
Sakshi Tyagi, Nirmal Singh, Jasleen kaur Virdi, Amteshwar Singh Jaggi
Journal of Physiology and Biochemistry.2019; 75(1): 19. CrossRef - Regulation of glucose metabolism by bioactive phytochemicals for the management of type 2 diabetes mellitus
Chao Zhao, Chengfeng Yang, Sydney Tang Chi Wai, Yanbo Zhang, Maria P. Portillo, Paolo Paoli, Yijing Wu, Wai San Cheang, Bin Liu, Christian Carpéné, Jianbo Xiao, Hui Cao
Critical Reviews in Food Science and Nutrition.2019; 59(6): 830. CrossRef - Pulse pressure amplification and cardiac autonomic dysfunction in patients with type 2 diabetes mellitus
Ioanna Eleftheriadou, George C. Drosos, Anastasios Tentolouris, Giorgios Konstantonis, Petros P. Sfikakis, Athanasios D. Protogerou, Nikolaos Tentolouris
Journal of Human Hypertension.2018; 32(8-9): 531. CrossRef - Exposure to hypoglycemia and risk of stroke
Logan Smith, Diya Chakraborty, Pallab Bhattacharya, Deepaneeta Sarmah, Sebastian Koch, Kunjan R. Dave
Annals of the New York Academy of Sciences.2018; 1431(1): 25. CrossRef - Association between the risk of falls and osteoporotic fractures in patients with type 2 diabetes mellitus
Maki Yokomoto-Umakoshi, Ippei Kanazawa, Shiori Kondo, Toshitsugu Sugimoto
Endocrine Journal.2017; 64(7): 727. CrossRef - Diabetes‐induced mechanophysiological changes in the esophagus
Jingbo Zhao, Hans Gregersen
Annals of the New York Academy of Sciences.2016; 1380(1): 139. CrossRef

 KDA
KDA PubReader
PubReader Cite
Cite