
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Review
- Drug/Regimen
- New, Novel Lipid-Lowering Agents for Reducing Cardiovascular Risk: Beyond Statins
- Kyuho Kim, Henry N. Ginsberg, Sung Hee Choi
- Diabetes Metab J. 2022;46(4):517-532. Published online July 27, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0198
- Correction in: Diabetes Metab J 2022;46(5):817
- 10,009 View
- 865 Download
- 25 Web of Science
- 25 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
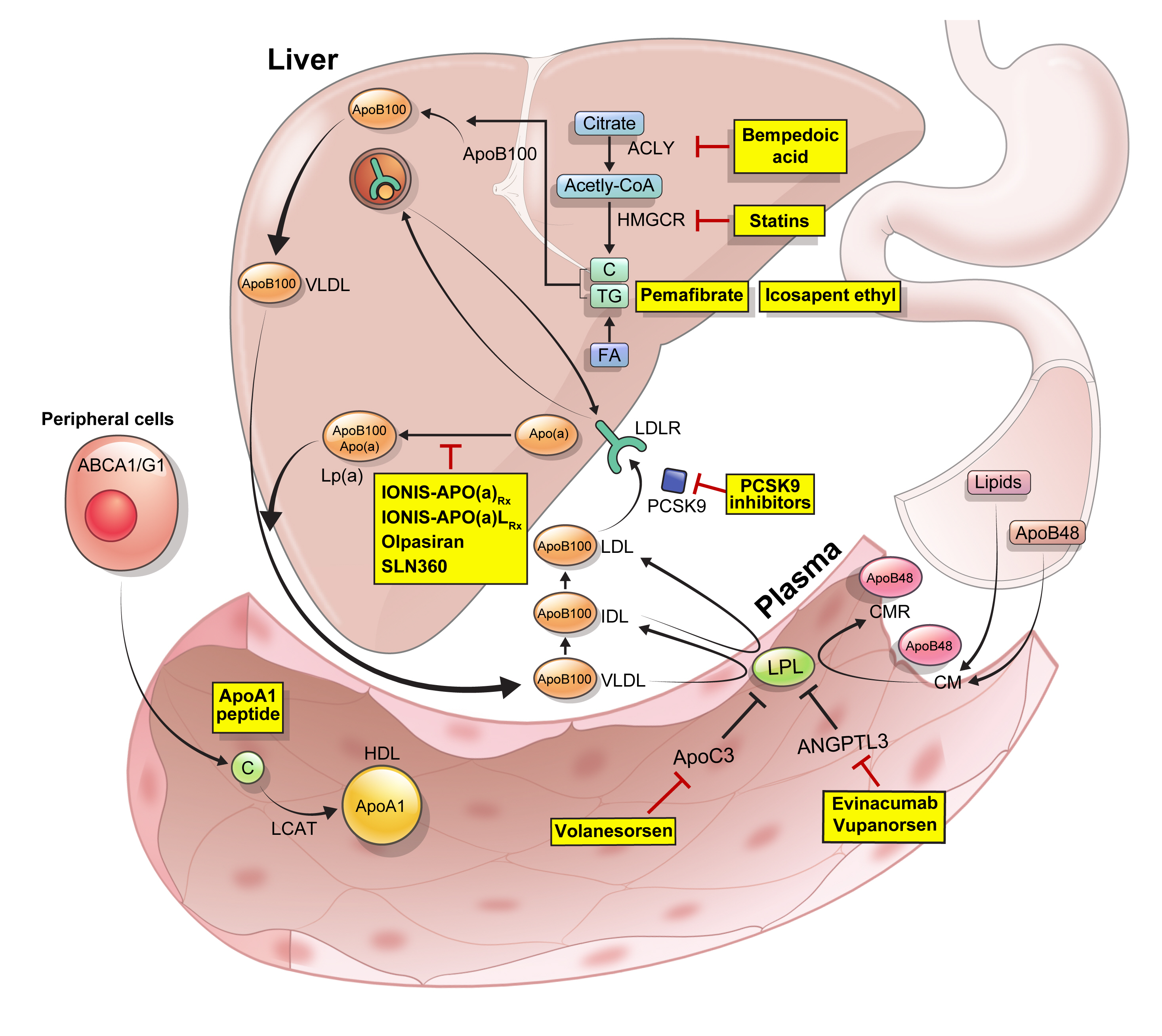
ePub - Statins are the cornerstone of the prevention and treatment of atherosclerotic cardiovascular disease (ASCVD). However, even under optimal statin therapy, a significant residual ASCVD risk remains. Therefore, there has been an unmet clinical need for novel lipid-lowering agents that can target low-density lipoprotein cholesterol (LDL-C) and other atherogenic particles. During the past decade, several drugs have been developed for the treatment of dyslipidemia. Inclisiran, a small interfering RNA that targets proprotein convertase subtilisin/kexin type 9 (PCSK9), shows comparable effects to that of PCSK9 monoclonal antibodies. Bempedoic acid, an ATP citrate lyase inhibitor, is a valuable treatment option for the patients with statin intolerance. Pemafibrate, the first selective peroxisome proliferator-activated receptor alpha modulator, showed a favorable benefit-risk balance in phase 2 trial, but the large clinical phase 3 trial (PROMINENT) was recently stopped for futility based on a late interim analysis. High dose icosapent ethyl, a modified eicosapentaenoic acid preparation, shows cardiovascular benefits. Evinacumab, an angiopoietin-like 3 (ANGPTL3) monoclonal antibody, reduces plasma LDL-C levels in patients with refractory hypercholesterolemia. Novel antisense oligonucleotides targeting apolipoprotein C3 (apoC3), ANGPTL3, and lipoprotein(a) have significantly attenuated the levels of their target molecules with beneficial effects on associated dyslipidemias. Apolipoprotein A1 (apoA1) is considered as a potential treatment to exploit the athero-protective effects of high-density lipoprotein cholesterol (HDL-C), but solid clinical evidence is necessary. In this review, we discuss the mode of action and clinical outcomes of these novel lipid-lowering agents beyond statins.
-
Citations
Citations to this article as recorded by- The role of adherence in patients with chronic diseases
Michel Burnier
European Journal of Internal Medicine.2024; 119: 1. CrossRef - Bempedoic acid: new evidence and recommendations on use
Kristina Paponja, Ivan Pećin, Željko Reiner, Maciej Banach
Current Opinion in Lipidology.2024; 35(1): 41. CrossRef - Genetic insights into repurposing statins for hyperthyroidism prevention: a drug-target Mendelian randomization study
Anqi Huang, Xinyi Wu, Jiaqi Lin, Chiju Wei, Wencan Xu
Frontiers in Endocrinology.2024;[Epub] CrossRef - Targeting host-specific metabolic pathways—opportunities and challenges for anti-infective therapy
Monika I. Konaklieva, Balbina J. Plotkin
Frontiers in Molecular Biosciences.2024;[Epub] CrossRef - Neutrophil Extracellular Traps (NETs) and Atherosclerosis: Does Hypolipidemic Treatment Have an Effect?
Petros Adamidis, Despoina Pantazi, Iraklis Moschonas, Evangelos Liberopoulos, Alexandros Tselepis
Journal of Cardiovascular Development and Disease.2024; 11(3): 72. CrossRef - Modulating effects of crocin on lipids and lipoproteins: Mechanisms and potential benefits
Habib Yaribeygi, Mina Maleki, Farin Rashid-Farrokhi, Payman Raise Abdullahi, Mohammad Amin Hemmati, Tannaz Jamialahmadi, Amirhossein Sahebkar
Heliyon.2024; 10(7): e28837. CrossRef - Assessing the Benefits of Lifestyle Influences on Cardiovascu-lar Health After Acute Coronary Syndrome
Marius Rus, Claudia Elena Stanis, Paula Marian, Lilliana Oana Pobirci, Loredana Ioana Banszki, Veronica Huplea, Gheorghe Adrian Osiceanu, Bianca-Maria Pop, Gabriela Dogaru, Felicia Liana Andronie-Cioara
Balneo and PRM Research Journal.2024; 15(Vol.15, no): 660. CrossRef - Liver cancer cells as the model for developing liver-targeted RNAi therapeutics
Beibei Hou, Linhui Qin, Linfeng Huang
Biochemical and Biophysical Research Communications.2023; 644: 85. CrossRef - Insights into Causal Cardiovascular Risk Factors from Mendelian Randomization
C. M. Schooling, J. V. Zhao
Current Cardiology Reports.2023; 25(2): 67. CrossRef - Secoisolariciresinol diglucoside and anethole ameliorate lipid abnormalities, oxidative injury, hypercholesterolemia, heart, and liver conditions
Sana Noreen, Habib‐ur Rehman, Tabussam Tufail, Huma Badar Ul Ain, Chinaza Godswill Awuchi
Food Science & Nutrition.2023; 11(6): 2620. CrossRef - Colesterol remanente, riesgo vascular y prevención de la arteriosclerosis
Xavier Pintó, Marta Fanlo, Virginia Esteve, Jesús Millán, Agustín Blanco, Mariano Blasco, José Luís Díaz Díaz, Ángel Díaz Rodríguez, Alipio Mangas, Vicente Pascual, Juan Pedro Botet, Pablo Pérez Martínez
Clínica e Investigación en Arteriosclerosis.2023; 35(4): 206. CrossRef - Evolving Management of Low‐Density Lipoprotein Cholesterol: A Personalized Approach to Preventing Atherosclerotic Cardiovascular Disease Across the Risk Continuum
Michael J. Wilkinson, Norman E. Lepor, Erin D. Michos
Journal of the American Heart Association.2023;[Epub] CrossRef - The cell origins of foam cell and lipid metabolism regulated by mechanical stress in atherosclerosis
Zhi Ouyang, Jian Zhong, Junyi Shen, Ye Zeng
Frontiers in Physiology.2023;[Epub] CrossRef - Triglyceride-Rich Lipoprotein Metabolism: Key Regulators of Their Flux
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(13): 4399. CrossRef - Remnant cholesterol, vascular risk, and prevention of atherosclerosis
Xavier Pintó, Marta Fanlo, Virginia Esteve, Jesús Millán
Clínica e Investigación en Arteriosclerosis (English Edition).2023; 35(4): 206. CrossRef - Antibiotics and Lipid-Modifying Agents: Potential Drug–Drug Interactions and Their Clinical Implications
Marios Spanakis, Danny Alon-Ellenbogen, Petros Ioannou, Nikolaos Spernovasilis
Pharmacy.2023; 11(4): 130. CrossRef - Advances in Treatment of Dyslipidemia
Jill Dybiec, Wiktoria Baran, Bartłomiej Dąbek, Piotr Fularski, Ewelina Młynarska, Ewa Radzioch, Jacek Rysz, Beata Franczyk
International Journal of Molecular Sciences.2023; 24(17): 13288. CrossRef - Peroxisome Proliferator-Activated Receptor α in Lipoprotein Metabolism and Atherosclerotic Cardiovascular Disease
Elena Valeria Fuior, Evangelia Zvintzou, Theodosios Filippatos, Katerina Giannatou, Victoria Mparnia, Maya Simionescu, Anca Violeta Gafencu, Kyriakos E. Kypreos
Biomedicines.2023; 11(10): 2696. CrossRef - Preparation, characterization and in vivo pharmacokinetic study of ginsenoside Rb1-PLGA nanoparticles
Lixin Du, Huiling Lu, Yifei Xiao, Zhihua Guo, Ya Li
Scientific Reports.2023;[Epub] CrossRef - Dysregulation of Cholesterol Homeostasis in Ovarian Cancer
Zahraa Qusairy, Anne Gangloff, Shuk On Annie Leung
Current Oncology.2023; 30(9): 8386. CrossRef - Riesgo residual. Conclusiones
Ángel Cequier, José Luis Zamorano
Revista Española de Cardiología Suplementos.2023; 23: 25. CrossRef - Causal effects of circulating lipids and lipid-lowering drugs on the risk of urinary stones: a Mendelian randomization study
Zilong Tan, Jing Hong, Aochuan Sun, Mengdi Ding, Jianwu Shen
Frontiers in Endocrinology.2023;[Epub] CrossRef - Bibliometric analysis of residual cardiovascular risk: trends and frontiers
Lin Wang, Sutong Wang, Chaoyuan Song, Yiding Yu, Yuehua Jiang, Yongcheng Wang, Xiao Li
Journal of Health, Population and Nutrition.2023;[Epub] CrossRef - Current Understanding on the Genetic Basis of Key Metabolic Disorders: A Review
Kenneth Francis Rodrigues, Wilson Thau Lym Yong, Md. Safiul Alam Bhuiyan, Shafiquzzaman Siddiquee, Muhammad Dawood Shah, Balu Alagar Venmathi Maran
Biology.2022; 11(9): 1308. CrossRef - Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
Joon Ho Moon, Kyuho Kim, Sung Hee Choi
Endocrinology and Metabolism.2022; 37(4): 575. CrossRef
- The role of adherence in patients with chronic diseases
Original Article
- Metabolic Phenotype of Glycogen Synthase Gene Inhibition in Human Skeletal Muscle Cells.
- Jae Joon Koh, Kyong Soo Park, Jeong Mi Kim, Seong Yeon Kim, Hong Kyu Lee, Theodore P Ciaraldi, Robert R Henry
- Korean Diabetes J. 2000;24(3):331-339. Published online January 1, 2001
- 905 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Glycogen synthase (GS) is the rate-limiting enzyme controlling non-oxidative glucose disposal in skeletal muscle. Reduction in GS activity and impaired insulin responsiveness are characteristic features of skeletal muscle in type 2 diabetes that contribute to glucose intolerance. These properties also exist in human skeletal muscle cell cultures from type 2 diabetic subjects. The aim of study is to determine the effect of an isolated reduction in GS on glucose metabolism and if this change can generate a diabetes-like state. METHODS: Cultured skeletal muscle cells from non-diabetic subjects were treated with antisense oligodeoxynucleotides (ODN) to GS to interfere with expression of the gene for 6 days. GS activity, protein expression, glycogen synthesis and cellular glycogen content were measured. RESULTS: Treatment with antisense ODN reduced GS protein expression by 70% compared to control (scrambled) ODN (p<0.01). Both total GS activity and that measured at 0.1 mM G-6-P were reduced by antisense ODN treatment. Insulin responsiveness of GS was also halved. Basal GS FV0.1 was decreased in both antisense ODN and control ODN treated cells and antisense treated cells did not show increase in GS FV0.1 in response to insulin stimulation. Glucose incorporation into glycogen under basal conditions was unaltered after antisense ODN treatment, though no further stimulation in response to insulin was observed. Yet both cellular glycogen content and glycogen synthesis were lower in antisense ODN treated cells compared to control ODN treated cells. CONCLUSIONS: Reduction in GS expression in human skeletal muscle cell impair GS activity and insulin responsiveness but does not replicate the abnormalities of glycogen synthesis found in cultured diabetic skeletal muscle cells.

 KDA
KDA
 First
First Prev
Prev





