
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Role of Intestinal Microbiota in Metabolism of Voglibose In Vitro and In Vivo
- Mahesh Raj Nepal, Mi Jeong Kang, Geon Ho Kim, Dong Ho Cha, Ju-Hyun Kim, Tae Cheon Jeong
- Diabetes Metab J. 2020;44(6):908-918. Published online April 6, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0147
- 5,676 View
- 114 Download
- 6 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
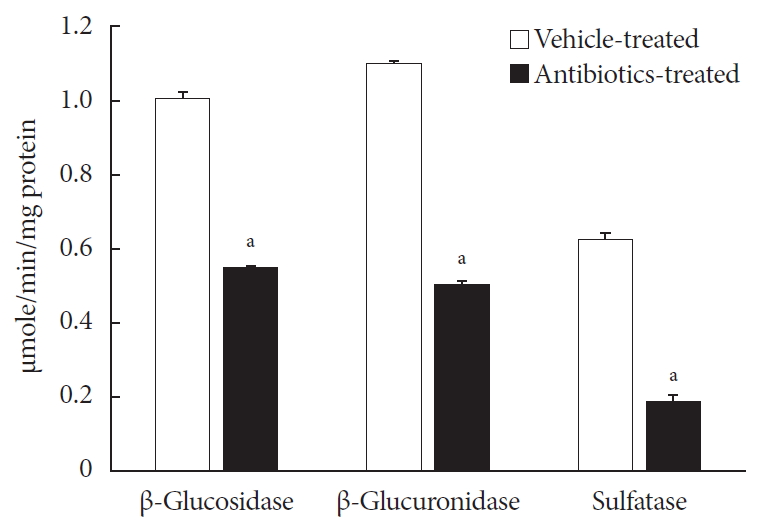
ePub Background Voglibose, an α-glucosidase inhibitor, inhibits breakdown of complex carbohydrates into simple sugar units in intestine. Studies showed that voglibose metabolism in the liver might be negligible due to its poor intestinal absorption. Numerous microorganisms live in intestine and have several roles in metabolism and detoxification of various xenobiotics. Due to the limited information, the possible metabolism of voglibose by intestinal microbiota was investigated
in vitro andin vivo .Methods For the
in vitro study, different concentrations of voglibose were incubated with intestinal contents, prepared from both vehicle- and antibiotics-treated mice, to determine the decreased amount of voglibose over time by using liquid chromatography-mass spectrometry. Similarly,in vivo pharmacodynamic effect of voglibose was determined following the administration of voglibose and starch in vehicle- and antibiotic-pretreated non-diabetic and diabetic mice, by measuring the modulatory effects of voglibose on blood glucose levels.Results The
in vitro results indicated that the remaining voglibose could be significantly decreased when incubated with the intestinal contents from normal mice compared to those from antibiotic-treated mice, which had less enzyme activities. Thein vivo results showed that the antibiotic pretreatment resulted in reduced metabolism of voglibose. This significantly lowered blood glucose levels in antibiotic-pretreated mice compared to the control animals.Conclusion The present results indicate that voglibose would be metabolized by the intestinal microbiota, and that this metabolism might be pharmacodynamically critical in lowering blood glucose levels in mice.
-
Citations
Citations to this article as recorded by- Pharmacomicrobiomics and type 2 diabetes mellitus: A novel perspective towards possible treatment
Liyang Jia, Shiqiong Huang, Boyu Sun, Yongguang Shang, Chunsheng Zhu
Frontiers in Endocrinology.2023;[Epub] CrossRef - Phenolics from endophytic fungi as natural α-glucosidase inhibitors: A comprehensive review
Muhammad Imran Tousif, Saba Tauseef, Sadeer Nabeelah, Jugreet Sharmeen, Gokhan Zengin, Lesetja Legoabe, Muhammad Imran, Mohamad Fawzi Mahomoodally
Journal of Molecular Structure.2023; 1291: 135852. CrossRef - Ligand-targeted fishing of α-glucosidase inhibitors from Tribulus terrestris L. based on chitosan-functionalized multi-walled carbon nanotubes with immobilized α-glucosidase
Xin Meng, Hou Zong, Zhong Zheng, Junpeng Xing, Zhiqiang Liu, Fengrui Song, Shu Liu
Analytical and Bioanalytical Chemistry.2023; 415(14): 2677. CrossRef - Isolation, structure elucidation, and biological activities of sesquiterpenes and phthalides from two edible mushrooms Pleurotus species
Jewel C De Padua, Emi Fukushima-Sakuno, Kotomi Ueno, Thomas Edison E dela Cruz, Atsushi Ishihara
Bioscience, Biotechnology, and Biochemistry.2023; 87(12): 1429. CrossRef - Effects of Oral Glucose-Lowering Agents on Gut Microbiota and Microbial Metabolites
Dongmei Wang, Jieying Liu, Liyuan Zhou, Qian Zhang, Ming Li, Xinhua Xiao
Frontiers in Endocrinology.2022;[Epub] CrossRef - 18:0 Lyso PC, a natural product with potential PPAR-γ agonistic activity, plays hypoglycemic effect with lower liver toxicity and cardiotoxicity in db/db mice
Yiming Ma, Xinyi Du, Dandan Zhao, Kegong Tang, Xiaona Wang, Shaoting Guo, Xiaobei Li, Song Mei, Na Sun, Jiaqi Liu, Chengyu Jiang
Biochemical and Biophysical Research Communications.2021; 579: 168. CrossRef
- Pharmacomicrobiomics and type 2 diabetes mellitus: A novel perspective towards possible treatment
- Clinical Diabetes & Therapeutics
- Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial
- Tae Jung Oh, Jae Myung Yu, Kyung Wan Min, Hyun Shik Son, Moon Kyu Lee, Kun Ho Yoon, Young Duk Song, Joong Yeol Park, In Kyung Jeong, Bong Soo Cha, Yong Seong Kim, Sei Hyun Baik, In Joo Kim, Doo Man Kim, Sung Rae Kim, Kwan Woo Lee, Jeong Hyung Park, In Kyu Lee, Tae Sun Park, Sung Hee Choi, Sung Woo Park
- Diabetes Metab J. 2019;43(3):276-286. Published online December 7, 2018
- DOI: https://doi.org/10.4093/dmj.2018.0051
- 7,057 View
- 99 Download
- 13 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Combination of metformin to reduce the fasting plasma glucose level and an α-glucosidase inhibitor to decrease the postprandial glucose level is expected to generate a complementary effect. We compared the efficacy and safety of a fixed-dose combination of voglibose plus metformin (vogmet) with metformin monotherapy in drug-naïve newly-diagnosed type 2 diabetes mellitus.
Methods A total of 187 eligible patients aged 20 to 70 years, with a glycosylated hemoglobin (HbA1c) level of 7.0% to 11.0%, were randomized into either vogmet or metformin treatments for 24 weeks. A change in the HbA1c level from baseline was measured at week 24.
Results The reduction in the levels of HbA1c was −1.62%±0.07% in the vogmet group and −1.31%±0.07% in the metformin group (
P =0.003), and significantly more vogmet-treated patients achieved the target HbA1c levels of <6.5% (P =0.002) or <7% (P =0.039). Glycemic variability was also significantly improved with vogmet treatment, estimated by M-values (P =0.004). Gastrointestinal adverse events and hypoglycemia (%) were numerically lower in the vogmet-treated group. Moreover, a significant weight loss was observed with vogmet treatment compared with metformin (−1.63 kg vs. −0.86 kg,P =0.039).Conclusion Vogmet is a safe antihyperglycemic agent that controls blood glucose level effectively, yields weight loss, and is superior to metformin in terms of various key glycemic parameters without increasing the risk of hypoglycemia.
-
Citations
Citations to this article as recorded by- Phytochemical analysis and antihyperglycemic activity of Castilleja arvensis
Mónica Aideé Díaz-Román, Juan José Acevedo-Fernández, Gabriela Ávila-Villarreal, Elizabeth Negrete-León, A. Berenice Aguilar-Guadarrama
Fitoterapia.2024; 174: 105839. CrossRef - YAP/TAZ axis was involved in the effects of metformin on breast cancer
Yu Xu, Hongke Cai, Yuanfeng Xiong, Li Tang, Longjiang Li, Li Zhang, Yi Shen, Yongqiang Yang, Ling Lin, Jiayi Huang
Journal of Chemotherapy.2023; 35(7): 627. CrossRef - Diabetes remission: Myth or reality?
Ashok Kumar, ShubhaLaxmi Margekar, Ravi Kumar
Indian Journal of Medical Specialities.2023; 14(1): 3. CrossRef - Analysis of Reports Sent to the Portuguese Pharmacovigilance System and Published Literature Regarding the Safety of Metformin in the Elderly
Beatriz Esteves, Cristina Monteiro, Ana Paula Coelho Duarte
Healthcare.2023; 11(15): 2197. CrossRef - Rapid prediction method of α-Glycosidase inhibitory activity of Coreopsis tinctoria extract from different habitats by near infrared spectroscopy
Xiaogang He, Xiang Han, Jiaping Yu, Yulong Feng, Ganghui Chu
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy.2022; 268: 120601. CrossRef - Insulin autoimmune syndrome in patients with type 2 diabetes: A report of two cases
Y. Shin, T.J. Oh, S.H. Choi, H.C. Jang
Diabetes & Metabolism.2021; 47(1): 101115. CrossRef - Efficacy and Safety of Treatment with Quadruple Oral Hypoglycemic Agents in Uncontrolled Type 2 Diabetes Mellitus: A Multi-Center, Retrospective, Observational Study
Jun Sung Moon, Sunghwan Suh, Sang Soo Kim, Heung Yong Jin, Jeong Mi Kim, Min Hee Jang, Kyung Ae Lee, Ju Hyung Lee, Seung Min Chung, Young Sang Lyu, Jin Hwa Kim, Sang Yong Kim, Jung Eun Jang, Tae Nyun Kim, Sung Woo Kim, Eonju Jeon, Nan Hee Cho, Mi-Kyung Ki
Diabetes & Metabolism Journal.2021; 45(5): 675. CrossRef - Quantifying Remission Probability in Type 2 Diabetes Mellitus
Sanjay Kalra, Ganapathi Bantwal, Nitin Kapoor, Rakesh Sahay, Saptarshi Bhattacharya, Beatrice Anne, Raju A Gopal, Sunil Kota, Ashok Kumar, Ameya Joshi, Debmalya Sanyal, Mangesh Tiwaskar, Ashok Kumar Das
Clinics and Practice.2021; 11(4): 850. CrossRef - The effect of voglibose on metabolic profiles in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of clinical trials
Peyman Nowrouzi-Sohrabi, Reza Tabrizi, Shahla Rezaei, Fatemeh Jafari, Kamran Hessami, Mehdi Abedi, Mohammad Jalali, Pedram Keshavarzi, Saeed Shahabi, Ali Asghar Kolahi, Kristin Carson-Chahhoud, Amirhossein Sahebkar, Saeid Safiri
Pharmacological Research.2020; 159: 104988. CrossRef - Role of Intestinal Microbiota in Metabolism of Voglibose In Vitro and In Vivo
Mahesh Raj Nepal, Mi Jeong Kang, Geon Ho Kim, Dong Ho Cha, Ju-Hyun Kim, Tae Cheon Jeong
Diabetes & Metabolism Journal.2020; 44(6): 908. CrossRef - Response: Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes metab J 2019;43;276-86)
Tae Jung Oh, Sung Hee Choi
Diabetes & Metabolism Journal.2019; 43(4): 547. CrossRef - Letter: Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2019;43;276-86)
Hannah Seok, Tae Seo Sohn
Diabetes & Metabolism Journal.2019; 43(4): 545. CrossRef
- Phytochemical analysis and antihyperglycemic activity of Castilleja arvensis
- Comparative Study about the Effects of Acarbose and Voglibose in Type 2 Diabetic Patients.
- In Kyung Jeong, Jae Hoon Chung, Yong Ki Min, Myung Shik Lee, Moon Kyu Lee, Kwang Won Kim, Yun Ey Chung, Joong Yeol Park, Sung Kwan Hong, Ki Up Lee
- Korean Diabetes J. 2002;26(2):134-145. Published online April 1, 2002
- 1,209 View
- 35 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Acarbose and voglibose are alpha-glucosidase inhibitors. Although different pharmacological effects and adverse abdominal events associated with the two drugs have been reported, no study directly compared acarbose and voglibose in diabetes has been undertaken. To compare the pharmacological effects and gastrointestinal adverse events between two drugs, a randomized, placebo-controlled, double-blind study was performed in type 2 diabetes patients. METHODS: The period of study was 12 weeks (observation period: 4 weeks; treatment period: 8 weeks). Fifty-three patients were randomized into two groups (the acarbose group: 24 patients; the voglibose group: 29 patients). The serum glucose, insulin, fructosamine, HbA1c, cholesterol, triglyceride and the incidence of adverse events were measured. RESULTS: 1) The reduction of glucose from before treatment to 4 weeks after treatment was significantly higher in the acarbose group, but the change before treatment and 8 weeks after treatment in the two groups was similar (p = 0.569). 2) The insulin significantly decreased after voglibose treatment (p = 0.040). 3) HbA1c level tended to decrease in voglibose group, and there was a significant decrease after acarbose treatment. However, the change in HbA1c level before and after treatment was similar between the two groups (p = 0.412). 4) The two drugs did not cause any other changes in the total, HDL-cholesterol and triglyceride. 5) The number of patients with gastrointestinal adverse events was significantly low 4 weeks after voglibose treatment (p = 0.049), but the incidence in the two groups was similar after 8 weeks (p = 0.215). CONCLUSIONS: Acarbose and voglibose significantly improved postprandial hyperglycemia in diabetes. The incidence of gastrointestinal adverse events was low 4 weeks after voglibose treatment.
- Lowering Effect of Voglibose, Monotherapy on Uncontrolled Postprandial Glucose in Patients with Non-Insulin Dependent Diabetes Mellitus (NIDDM) Being Treated with Strict Diet Control: Multicenter Open-Study.
- Jeong Taek Woo, Young Seol Kim, Young Kil Choi, Jin Woo Kim, In Myung Yang, Sung Woon Kim, Deog Yoon Kim, Kwang Won Kim, Moon Kyu Lee, Myung Shik Lee, Jae Hoon Jung, Kyu Jeong Ahn, Hyun Chul Lee, Young Deuk Song, Bong Soo Cha, Jee Hyun Lee, Hyung Joon Won
- Korean Diabetes J. 1998;22(3):419-428. Published online January 1, 2001
- 1,215 View
- 24 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
It is sometimes very difficult to control the elevation of postprandial glucose with diet therapy only in patients with NIDDM partly because of their defective insulin response to glucose. Recently the alpha-glucosidase inhibitors which inhibit carbohydrate digestion and suppress or delay absorption of the final breakdown products, glucose and fructose when it is taken orally with meal have been widely used in the treatment of diabetes. The drugs, however, provoke the adverse effects e.g. flatulence, diarrhea etc. in some patients. Therefore we studied the efficacy of the more recently developed alpha glucosidase inhibitor, Voglibose (Basen, Cheiljedang) METHODS: Fifty five patients whose postprandial two hour serum glucose levels were more than 11.1 mmol/L despite the strict diet therapy during the 4 week observation period were assigned to receive Voglibose 0.2 mg before each meal t.i.d. for 8 weeks. Of 55 subjects, 41 were given Voglibose 0.3 mg t..i.d. for the last 4 weeks because of their poor glucose control, RESULTS: The postprandial one and two hour serum glucose levels significantly decreased after therapy; 1 hour: 17.5+4.4 mmol/L(prior to therapy), 15.4+3.8 mmol/L(4 week after), 14.8+5.1 mmol/L(8 week), p <0.00l, 2 hour: 16.7+4.5 mmol/L, 14.8+3.9 mmol/ L, 14.8+4.5 mmol/L, p<0.00 l, t-tests for paired samples. Total serum cholesterol and HDL cholesterol levels also significantly decreased(5.24+1.06 - 4.90+1.27 mmol/L, p=0.036, 1.34+0.66 1.16 +0.3l mmol/L, p=0.035 respectively) However, HbAlc, serum fructosamine, insulin and triglyceride levels were not significantly changed. The prevalence of the adverse effects due to Voglibose was 14%(10/71). All of them were less than grade II of WHO criteria and disappeared despite continuing therapy. CONCLUSION: Voglibose monotherapy is considered as having an glucose lowering effect in patients with NIDDM whose adequate postprandial blood glucose cannot be achieved with diet therapy only.

 KDA
KDA
 First
First Prev
Prev





