
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- N6-Methyladenosine Methyltransferase METTL3 Alleviates Diabetes-Induced Testicular Damage through Modulating TUG1/Clusterin Axis
- Yuan Tian, Yue-Hai Xiao, Chao Sun, Bei Liu, Fa Sun
- Diabetes Metab J. 2023;47(2):287-300. Published online January 19, 2023
- DOI: https://doi.org/10.4093/dmj.2021.0306
- 2,207 View
- 153 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
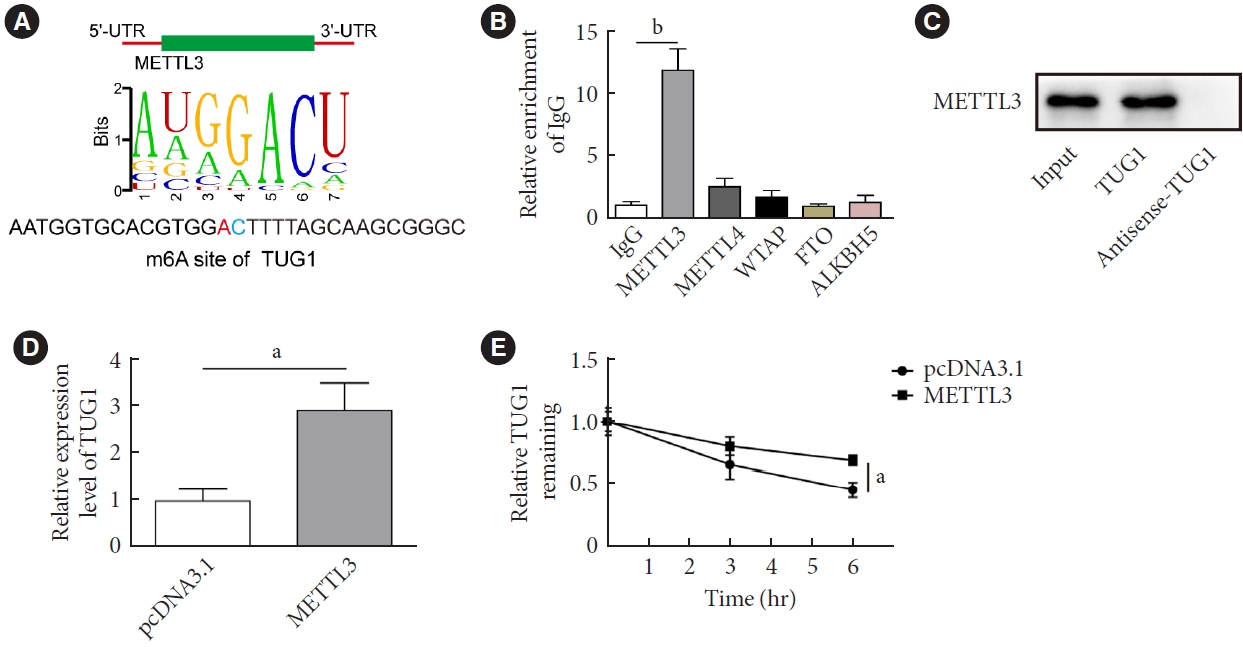
The present study investigated the regulatory effects of N6-methyladenosine (m6A) methyltransferase like-3 (METTL3) in diabetes-induced testicular damage.
Methods
In vivo diabetic mice and high glucose (HG) treated GC-1 spg cells were established. The mRNA and protein expressions were determined by real-time quantitative polymerase chain reaction, Western blot, immunofluorescence and immunohistochemistry staining. Levels of testosterone, blood glucose, cell viability, and apoptosis were detected by enzyme-linked immunosorbent assay, MTT, and flow cytometry, respectively. Molecular interactions were verified by RNA immunoprecipitation and RNA pull-down assay. Histopathological staining was performed to evaluate testicular injury.
Results
METTL3 and long non-coding RNA taurine up-regulated 1 (lncRNA TUG1) were downregulated in testicular tissues of diabetic mice and HG-treated GC-1 spg cells. METTL3 overexpression could reduce the blood glucose level, oxidative stress and testicular damage but enhance testosterone secretion in diabetic mouse model and HG-stimulated GC-1 spg cells. Mechanically, METTL3-mediated m6A methylation enhanced the stability of TUG1, then stabilizing the clusterin mRNA via recruiting serine and arginine rich splicing factor 1. Moreover, inhibition of TUG1/clusterin signaling markedly reversed the protective impacts of METTL3 overexpression on HG-stimulated GC-1 spg cells.
Conclusion
This study demonstrated that METTL3 ameliorated diabetes-induced testicular damage by upregulating the TUG1/clusterin signaling. These data further elucidate the potential regulatory mechanisms of m6A modification on diabetes-induced testicular injury. -
Citations
Citations to this article as recorded by- Negative Regulation of LINC01013 by METTL3 and YTHDF2 Enhances the Osteogenic Differentiation of Senescent Pre‐Osteoblast Cells Induced by Hydrogen Peroxide
Jiaxin Song, Yuejun Wang, Zhao Zhu, Wanqing Wang, Haoqing Yang, Zhaochen Shan
Advanced Biology.2024;[Epub] CrossRef - Diabetes and diabetic associative diseases: An overview of epigenetic regulations of TUG1
Mohammed Ageeli Hakami
Saudi Journal of Biological Sciences.2024; 31(5): 103976. CrossRef
- Negative Regulation of LINC01013 by METTL3 and YTHDF2 Enhances the Osteogenic Differentiation of Senescent Pre‐Osteoblast Cells Induced by Hydrogen Peroxide
- Pathophysiology
- Endoplasmic Reticulum Stress and Dysregulated Autophagy in Human Pancreatic Beta Cells
- Seoil Moon, Hye Seung Jung
- Diabetes Metab J. 2022;46(4):533-542. Published online July 27, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0070
- 4,537 View
- 250 Download
- 8 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
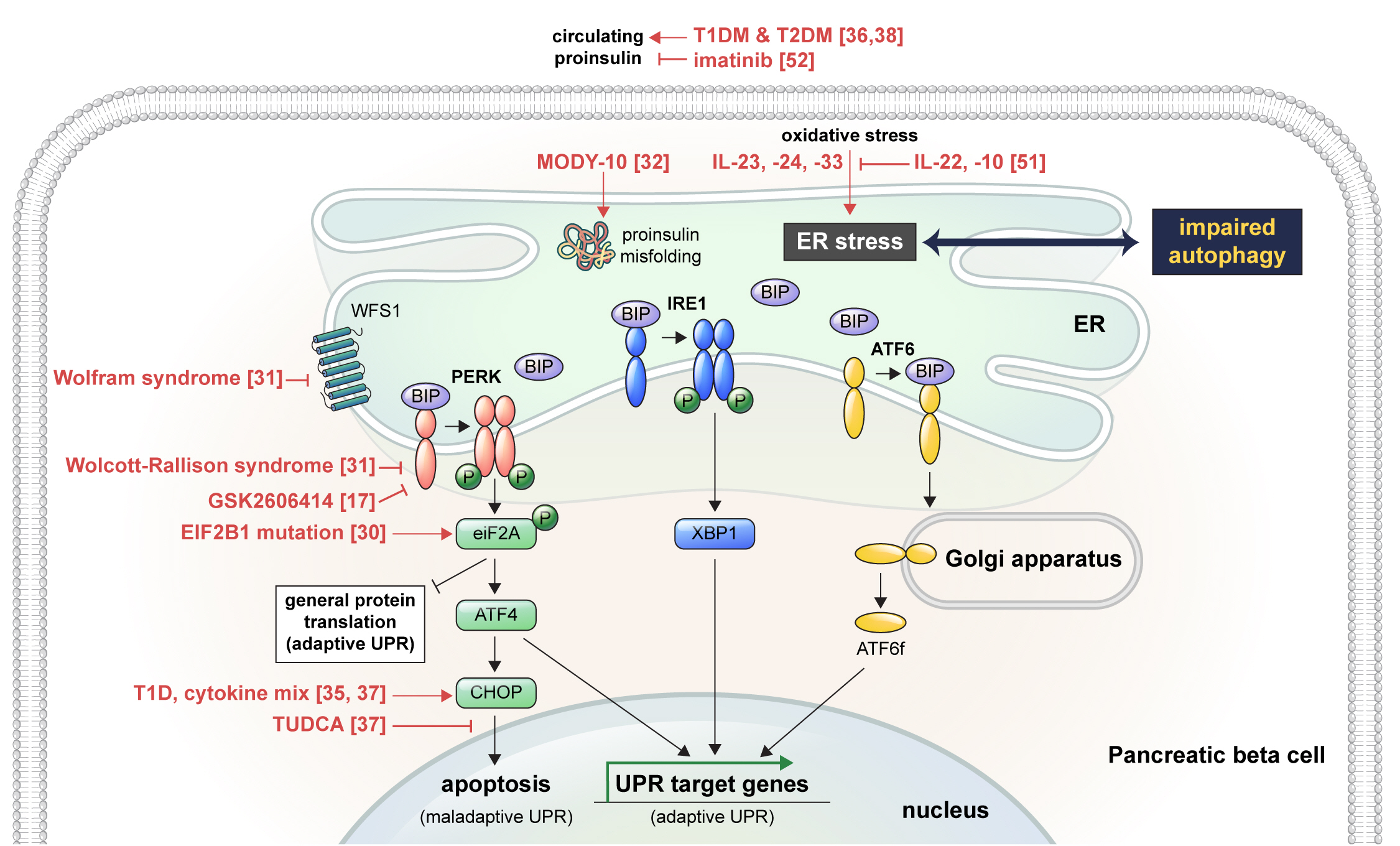
ePub - Pancreatic beta cell homeostasis is crucial for the synthesis and secretion of insulin; disruption of homeostasis causes diabetes, and is a treatment target. Adaptation to endoplasmic reticulum (ER) stress through the unfolded protein response (UPR) and adequate regulation of autophagy, which are closely linked, play essential roles in this homeostasis. In diabetes, the UPR and autophagy are dysregulated, which leads to beta cell failure and death. Various studies have explored methods to preserve pancreatic beta cell function and mass by relieving ER stress and regulating autophagic activity. To promote clinical translation of these research results to potential therapeutics for diabetes, we summarize the current knowledge on ER stress and autophagy in human insulin-secreting cells.
-
Citations
Citations to this article as recorded by- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
Seoil Moon, Ji Yoon Lim, Mirang Lee, Youngmin Han, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Mi Na Kim, Kyong Soo Park, Hye Seung Jung
Diabetes & Metabolism Journal.2024; 48(2): 231. CrossRef - Endoplasmic reticulum stress: A possible connection between intestinal inflammation and neurodegenerative disorders
Giorgio Vivacqua, Romina Mancinelli, Stefano Leone, Rosa Vaccaro, Ludovica Garro, Simone Carotti, Ludovica Ceci, Paolo Onori, Luigi Pannarale, Antonio Franchitto, Eugenio Gaudio, Arianna Casini
Neurogastroenterology & Motility.2024;[Epub] CrossRef - Docosahexanoic Acid Attenuates Palmitate-Induced Apoptosis by Autophagy Upregulation via GPR120/mTOR Axis in Insulin-Secreting Cells
Seok-Woo Hong, Jinmi Lee, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2024; 39(2): 353. CrossRef - Pancreatic islet remodeling in cotadutide-treated obese mice
Renata Spezani, Thatiany Souza Marinho, Luiz E. Macedo Cardoso, Marcia Barbosa Aguila, Carlos Alberto Mandarim-de-Lacerda
Life Sciences.2023; 327: 121858. CrossRef - Modulation of Unfolded Protein Response Restores Survival and Function of β-Cells Exposed to the Endocrine Disruptor Bisphenol A
Laura Maria Daian, Gabriela Tanko, Andrei Mircea Vacaru, Luiza Ghila, Simona Chera, Ana-Maria Vacaru
International Journal of Molecular Sciences.2023; 24(3): 2023. CrossRef - Interplay of skeletal muscle and adipose tissue: sarcopenic obesity
Min Jeong Park, Kyung Mook Choi
Metabolism.2023; 144: 155577. CrossRef - Identification and analysis of type 2 diabetes-mellitus-associated autophagy-related genes
Kun Cui, Zhizheng Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Sestrin2 in diabetes and diabetic complications
Xiaodan Zhang, Zirui Luo, Jiahong Li, Yaxuan Lin, Yu Li, Wangen Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Crosstalk between autophagy and insulin resistance: evidence from different tissues
Asie Sadeghi, Maryam Niknam, Mohammad Amin Momeni-Moghaddam, Maryam Shabani, Hamid Aria, Alireza Bastin, Maryam Teimouri, Reza Meshkani, Hamed Akbari
European Journal of Medical Research.2023;[Epub] CrossRef - Beta cell lipotoxicity in the development of type 2 diabetes: the need for species-specific understanding
Patricia Thomas, Meurig T. Gallagher, Gabriela Da Silva Xavier
Frontiers in Endocrinology.2023;[Epub] CrossRef
- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
- Complications
- Pathophysiologic Mechanisms and Potential Biomarkers in Diabetic Kidney Disease
- Chan-Young Jung, Tae-Hyun Yoo
- Diabetes Metab J. 2022;46(2):181-197. Published online March 24, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0329
- 11,988 View
- 789 Download
- 41 Web of Science
- 45 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Although diabetic kidney disease (DKD) remains the leading cause of end-stage kidney disease eventually requiring chronic kidney replacement therapy, the prevalence of DKD has failed to decline over the past 30 years. In order to reduce disease prevalence, extensive research has been ongoing to improve prediction of DKD onset and progression. Although the most commonly used markers of DKD are albuminuria and estimated glomerular filtration rate, their limitations have encouraged researchers to search for novel biomarkers that could improve risk stratification. Considering that DKD is a complex disease process that involves several pathophysiologic mechanisms such as hyperglycemia induced inflammation, oxidative stress, tubular damage, eventually leading to kidney damage and fibrosis, many novel biomarkers that capture one specific mechanism of the disease have been developed. Moreover, the increasing use of high-throughput omic approaches to analyze biological samples that include proteomics, metabolomics, and transcriptomics has emerged as a strong tool in biomarker discovery. This review will first describe recent advances in the understanding of the pathophysiology of DKD, and second, describe the current clinical biomarkers for DKD, as well as the current status of multiple potential novel biomarkers with respect to protein biomarkers, proteomics, metabolomics, and transcriptomics.
-
Citations
Citations to this article as recorded by- Role of polyphenols in the management of diabetic complications
Jeevika Raina, Atika Firdous, Gurvinder Singh, Rajesh Kumar, Charanjit Kaur
Phytomedicine.2024; 122: 155155. CrossRef - Role of MCP-1 as an inflammatory biomarker in nephropathy
Yanlong Liu, Ke Xu, Yuhua Xiang, Boyan Ma, Hailong Li, Yuan Li, Yue Shi, Shuju Li, Yan Bai
Frontiers in Immunology.2024;[Epub] CrossRef - Urinary podocyte stress marker as a prognostic indicator for diabetic kidney disease
Lingfeng Zeng, Jack Kit-Chung Ng, Winston Wing-Shing Fung, Gordon Chun-Kau Chan, Kai-Ming Chow, Cheuk-Chun Szeto
BMC Nephrology.2024;[Epub] CrossRef - Identification and validation of immune and cuproptosis - related genes for diabetic nephropathy by WGCNA and machine learning
Yubing Chen, Lijuan Liao, Baoju Wang, Zhan Wu
Frontiers in Immunology.2024;[Epub] CrossRef - Specific Alternation of Gut Microbiota and the Role of Ruminococcus gnavus in the Development of Diabetic Nephropathy
Jinni Hong, Tingting Fu, Weizhen Liu, Yu Du, Junmin Bu, Guojian Wei, Miao Yu, Yanshan Lin, Cunyun Min, Datao Lin
Journal of Microbiology and Biotechnology.2024; 34(3): 547. CrossRef - A Narrative Review of New Treatment Options for Diabetic Nephropathy
Aadhira Pillai, Darshna Fulmali
Cureus.2023;[Epub] CrossRef - Bamboo leaf: A review of traditional medicinal property, phytochemistry, pharmacology, and purification technology
Yaqian Cheng, Siqi Wan, Linna Yao, Ding Lin, Tong Wu, Yongjian Chen, Ailian Zhang, Chenfei Lu
Journal of Ethnopharmacology.2023; 306: 116166. CrossRef - Molecular Pathways of Diabetic Kidney Disease Inferred from Proteomics
Lan Wei, Yuanyuan Han, Chao Tu
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 117. CrossRef - Omics and Artificial Intelligence in Kidney Diseases
Nadja Grobe, Josef Scheiber, Hanjie Zhang, Christian Garbe, Xiaoling Wang
Advances in Kidney Disease and Health.2023; 30(1): 47. CrossRef - Intestinal microbiome diversity of diabetic and non-diabetic kidney disease: Current status and future perspective
Soumik Das, Ramanathan Gnanasambandan
Life Sciences.2023; 316: 121414. CrossRef - Pediatric Diabetic Nephropathy: Novel Insights from microRNAs
Francesca Lanzaro, Annalisa Barlabà, Angelica De Nigris, Federica Di Domenico, Valentina Verde, Emanuele Miraglia del Giudice, Anna Di Sessa
Journal of Clinical Medicine.2023; 12(4): 1447. CrossRef - Novel Biomarkers of Diabetic Kidney Disease
Jorge Rico-Fontalvo, Gustavo Aroca-Martínez, Rodrigo Daza-Arnedo, José Cabrales, Tomás Rodríguez-Yanez, María Cardona-Blanco, Juan Montejo-Hernández, Dairo Rodelo Barrios, Jhonny Patiño-Patiño, Elber Osorio Rodríguez
Biomolecules.2023; 13(4): 633. CrossRef - Diabetic vascular diseases: molecular mechanisms and therapeutic strategies
Yiwen Li, Yanfei Liu, Shiwei Liu, Mengqi Gao, Wenting Wang, Keji Chen, Luqi Huang, Yue Liu
Signal Transduction and Targeted Therapy.2023;[Epub] CrossRef - Metabolic phenotypes and risk of end-stage kidney disease in patients with type 2 diabetes
Lijun Zhao, Yutong Zou, Yucheng Wu, Linli Cai, Yuancheng Zhao, Yiting Wang, Xiang Xiao, Qing Yang, Jia Yang, Honghong Ren, Nanwei Tong, Fang Liu
Frontiers in Endocrinology.2023;[Epub] CrossRef - Identification of a New RNA and Protein Integrated Biomarker Panel Associated with Kidney Function Impairment in DKD: Translational Implications
Alessandra Scamporrino, Stefania Di Mauro, Agnese Filippello, Grazia Di Marco, Antonino Di Pino, Roberto Scicali, Maurizio Di Marco, Emanuele Martorana, Roberta Malaguarnera, Francesco Purrello, Salvatore Piro
International Journal of Molecular Sciences.2023; 24(11): 9412. CrossRef - Increased serum PCSK9 levels are associated with renal function impairment in patients with type 2 diabetes mellitus
Zhicai Feng, Xiangyu Liao, Hao Zhang, Juan Peng, Zhijun Huang, Bin Yi
Renal Failure.2023;[Epub] CrossRef - Analysis of Serum Pyrodeath Re-lated Proteins and Renal Injury in Patients with Type 2 DKD
茹洁 马
Asian Case Reports in Emergency Medicine.2023; 11(02): 53. CrossRef - Loganin reduces diabetic kidney injury by inhibiting the activation of NLRP3 inflammasome-mediated pyroptosis
Xiangri Kong, Yunyun Zhao, Xingye Wang, Yongjiang Yu, Ying Meng, Guanchi Yan, Miao Yu, Lihong Jiang, Wu Song, Bingmei Wang, Xiuge Wang
Chemico-Biological Interactions.2023; 382: 110640. CrossRef - Machine-learning algorithm-based prediction of a diagnostic model based on oxidative stress-related genes involved in immune infiltration in diabetic nephropathy patients
Heng-Mei Zhu, Na Liu, Dong-Xuan Sun, Liang Luo
Frontiers in Immunology.2023;[Epub] CrossRef - The roles of gut microbiota and its metabolites in diabetic nephropathy
Hui Zhao, Cheng-E Yang, Tian Liu, Ming-Xia Zhang, Yan Niu, Ming Wang, Jun Yu
Frontiers in Microbiology.2023;[Epub] CrossRef - High triglyceride levels increase the risk of diabetic microvascular complications: a cross-sectional study
Jiahang Li, Lei Shi, Guohong Zhao, Fei Sun, Zhenxing Nie, Zhongli Ge, Bin Gao, Yan Yang
Lipids in Health and Disease.2023;[Epub] CrossRef - Correlation of Kidney Injury Molecule-1 and Nephrin Levels in Iraqi Patients with Diabetic Nephropathy
Raghda Hisham Aljorani, Eman Saadi Saleh , Khalaf Gata Hussein Al Mohammadawi
Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 ).2023; 5: 99. CrossRef - Diabetic Nephropathy: Significance of Determining Oxidative Stress and Opportunities for Antioxidant Therapies
Marina Darenskaya, Sergey Kolesnikov, Natalya Semenova, Lyubov Kolesnikova
International Journal of Molecular Sciences.2023; 24(15): 12378. CrossRef - Evaluation of Neutrophil/Lymphocyte Ratio, Low-Density Lipoprotein/Albumin Ratio, and Red Cell Distribution Width/Albumin Ratio in the Estimation of Proteinuria in Uncontrolled Diabetic Patients
Duygu Tutan, Murat Doğan
Cureus.2023;[Epub] CrossRef - Hedysarum polybotrys polysaccharide attenuates renal inflammatory infiltration and fibrosis in diabetic mice by inhibiting the HMGB1/RAGE/TLR4 pathway
Changqing Xu, Yanxu Cheng, Zongmei Liu, Xiaoyan Fu
Experimental and Therapeutic Medicine.2023;[Epub] CrossRef - Abdominal adipose tissue and type 2 diabetic kidney disease: adipose radiology assessment, impact, and mechanisms
Fei Lu, Jinlei Fan, Fangxuan Li, Lijing Liu, Zhiyu Chen, Ziyu Tian, Liping Zuo, Dexin Yu
Abdominal Radiology.2023; 49(2): 560. CrossRef - Inhibition of MD2 by natural product-drived JM-9 attenuates renal inflammation and diabetic nephropathy in mice
Minxiu Wang, Qianhui Zhang, Shuaijie Lou, Leiming Jin, Gaojun Wu, Wenqi Wu, Qidong Tang, Yi Wang, Xiaohong Long, Ping Huang, Wu Luo, Guang Liang
Biomedicine & Pharmacotherapy.2023; 168: 115660. CrossRef - Multifaceted relationship between diabetes and kidney diseases: Beyond diabetes
Pasquale Esposito, Daniela Picciotto, Francesca Cappadona, Francesca Costigliolo, Elisa Russo, Lucia Macciò, Francesca Viazzi
World Journal of Diabetes.2023; 14(10): 1450. CrossRef - Mitochondrial antiviral signaling protein: a potential therapeutic target in renal disease
Meng Wu, Zhiyin Pei, Guangfeng Long, Hongbing Chen, Zhanjun Jia, Weiwei Xia
Frontiers in Immunology.2023;[Epub] CrossRef - Research progress on multiple cell death pathways of podocytes in diabetic kidney disease
Can Yang, Zhen Zhang, Jieting Liu, Peijian Chen, Jialing Li, Haiying Shu, Yanhui Chu, Luxin Li
Molecular Medicine.2023;[Epub] CrossRef - Quantitative profiling of carboxylic compounds by gas chromatography-mass spectrometry for revealing biomarkers of diabetic kidney disease
Rongrong Zhu, Yan Yuan, Rourou Qi, Jianying Liang, Yan Shi, Hongbo Weng
Journal of Chromatography B.2023; 1231: 123930. CrossRef - Jiangtang Decoction Ameliorates Diabetic Kidney Disease Through the Modulation of the Gut Microbiota
Jinni Hong, Tingting Fu, Weizhen Liu, Yu Du, Junmin Bu, Guojian Wei, Miao Yu, Yanshan Lin, Cunyun Min, Datao Lin
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 3707. CrossRef - GLP-1RA Combined with SGLT2 Inhibitors for the Treatment of Diabetic Kidney Disease: A Meta Analysis
莹 郭
Advances in Clinical Medicine.2023; 13(11): 18117. CrossRef - Potential application of Klotho as a prognostic biomarker for patients with diabetic kidney disease: a meta-analysis of clinical studies
Li Xia Yu, Min Yue Sha, Yue Chen, Fang Tan, Xi Liu, Shasha Li, Qi-Feng Liu
Therapeutic Advances in Chronic Disease.2023;[Epub] CrossRef - Single-Cell RNA Sequencing Reveals RAC1 Involvement in Macrophages Efferocytosis in Diabetic Kidney Disease
Yi Song, Yifan Liu, Feng Guo, Lin Zhao, Guijun Qin
Inflammation.2023;[Epub] CrossRef - Research progress of natural active compounds on improving podocyte function to reduce proteinuria in diabetic kidney disease
Le Gong, Rui Wang, Xinyu Wang, Jing Liu, Zhaodi Han, Qian Li, Yi Jin, Hui Liao
Renal Failure.2023;[Epub] CrossRef - Identification of potential crosstalk genes and mechanisms between periodontitis and diabetic nephropathy through bioinformatic analysis
Huijuan Lu, Jia Sun, Jieqiong Sun
Medicine.2023; 102(52): e36802. CrossRef - Mitochondrial RNAs as Potential Biomarkers of Functional Impairment in Diabetic Kidney Disease
Stefania Di Mauro, Alessandra Scamporrino, Agnese Filippello, Maurizio Di Marco, Maria Teresa Di Martino, Francesca Scionti, Antonino Di Pino, Roberto Scicali, Roberta Malaguarnera, Francesco Purrello, Salvatore Piro
International Journal of Molecular Sciences.2022; 23(15): 8198. CrossRef - Renoprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors: Focusing on Renal Hemodynamics
Nam Hoon Kim, Nan Hee Kim
Diabetes & Metabolism Journal.2022; 46(4): 543. CrossRef - Partial Synthetic PPARƳ Derivative Ameliorates Aorta Injury in Experimental Diabetic Rats Mediated by Activation of miR-126-5p Pi3k/AKT/PDK 1/mTOR Expression
Yasmin M. Ahmed, Raha Orfali, Nada S. Abdelwahab, Hossam M. Hassan, Mostafa E. Rateb, Asmaa M. AboulMagd
Pharmaceuticals.2022; 15(10): 1175. CrossRef - Polydatin attenuates tubulointerstitial fibrosis in diabetic kidney disease by inhibiting YAP expression and nuclear translocation
Manlin He, Lan Feng, Yang Chen, Bin Gao, Yiwei Du, Lu Zhou, Fei Li, Hongbao Liu
Frontiers in Physiology.2022;[Epub] CrossRef - Prevalence of diabetic nephropathy in the diabetes mellitus population: A protocol for systematic review and meta-analysis
Sicheng Li, Huidi Xie, Yang Shi, Hongfang Liu
Medicine.2022; 101(42): e31232. CrossRef - Stratification of diabetic kidney diseases via data-independent acquisition proteomics–based analysis of human kidney tissue specimens
Qinghua Huang, Xianming Fei, Zhaoxian Zhong, Jieru Zhou, Jianguang Gong, Yuan Chen, Yiwen Li, Xiaohong Wu
Frontiers in Endocrinology.2022;[Epub] CrossRef - Novel biomarkers and therapeutic approaches for diabetic retinopathy and nephropathy: Recent progress and future perspectives
Ziyan Xie, Xinhua Xiao
Frontiers in Endocrinology.2022;[Epub] CrossRef - Diabetic Kidney Disease
Susanne B. Nicholas, Amy K. Mottl
Nephrology Self-Assessment Program.2022; 21(5): 394. CrossRef
- Role of polyphenols in the management of diabetic complications
- Basic Research
- Hypoxia Increases β-Cell Death by Activating Pancreatic Stellate Cells within the Islet
- Jong Jin Kim, Esder Lee, Gyeong Ryul Ryu, Seung-Hyun Ko, Yu-Bae Ahn, Ki-Ho Song
- Diabetes Metab J. 2020;44(6):919-927. Published online May 11, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0181
- 5,935 View
- 146 Download
- 15 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
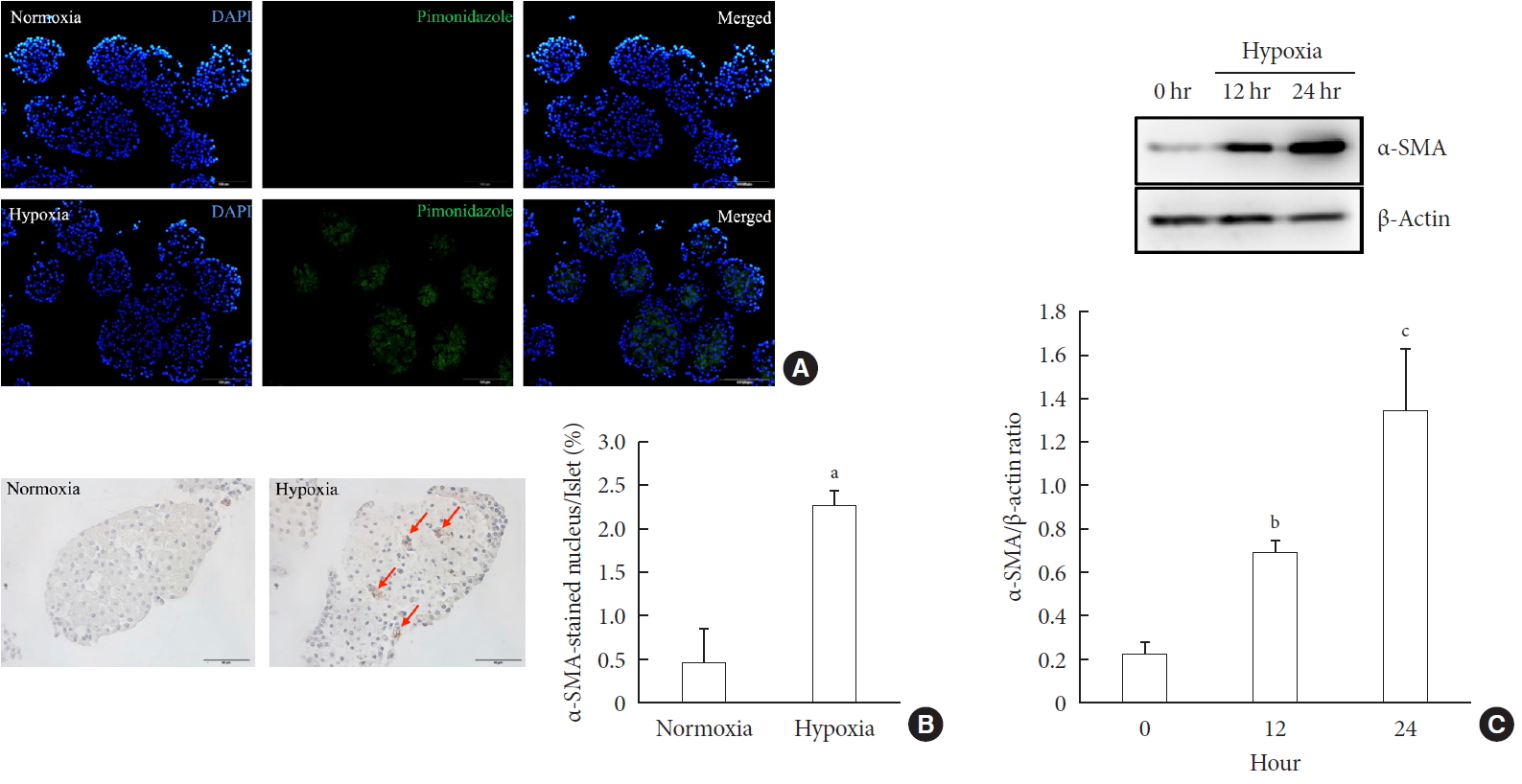
ePub Background Hypoxia can occur in pancreatic islets in type 2 diabetes mellitus. Pancreatic stellate cells (PSCs) are activated during hypoxia. Here we aimed to investigate whether PSCs within the islet are also activated in hypoxia, causing β-cell injury.
Methods Islet and primary PSCs were isolated from Sprague Dawley rats, and cultured in normoxia (21% O2) or hypoxia (1% O2). The expression of α-smooth muscle actin (α-SMA), as measured by immunostaining and Western blotting, was used as a marker of PSC activation. Conditioned media (hypoxia-CM) were obtained from PSCs cultured in hypoxia.
Results Islets and PSCs cultured in hypoxia exhibited higher expressions of α-SMA than did those cultured in normoxia. Hypoxia increased the production of reactive oxygen species. The addition of N-acetyl-L-cysteine, an antioxidant, attenuated the hypoxia-induced PSC activation in islets and PSCs. Islets cultured in hypoxia-CM showed a decrease in cell viability and an increase in apoptosis.
Conclusion PSCs within the islet are activated in hypoxia through oxidative stress and promote islet cell death, suggesting that hypoxia-induced PSC activation may contribute to β-cell loss in type 2 diabetes mellitus.
-
Citations
Citations to this article as recorded by- Effects of hypoxia in the diabetic corneal stroma microenvironment
Purnima Sharma, Jian-Xing Ma, Dimitrios Karamichos
Experimental Eye Research.2024; 240: 109790. CrossRef - Visualizing hypoxic modulation of beta cell secretions via a sensor augmented oxygen gradient
Kai Duan, Mengyang Zhou, Yong Wang, Jose Oberholzer, Joe F. Lo
Microsystems & Nanoengineering.2023;[Epub] CrossRef - Pancreatic stellate cells promote pancreatic β-cell death through exosomal microRNA transfer in hypoxia
Esder Lee, Gyeong Ryul Ryu, Seung-Hyun Ko, Yu-Bae Ahn, Ki-Ho Song
Molecular and Cellular Endocrinology.2023; 572: 111947. CrossRef - Pancreatic stellate cells in pancreatic cancer: as potential targets for future therapy
Zhengfeng Wang, Ru He, Shi Dong, Wence Zhou
Frontiers in Oncology.2023;[Epub] CrossRef - Recent advances in the development of bioartificial pancreas using 3D bioprinting for the treatment of type 1 diabetes: a review
Anushikha Ghosh, Arka Sanyal, Abhik Mallick
Exploration of Medicine.2023; : 886. CrossRef - Pancreas and islet morphology in cystic fibrosis: clues to the etiology of cystic fibrosis-related diabetes
Sarah S. Malik, Diksha Padmanabhan, Rebecca L. Hull-Meichle
Frontiers in Endocrinology.2023;[Epub] CrossRef - Diabetic mellitus, vascular calcification and hypoxia: A complex and neglected tripartite relationship
Xue-Jiao Sun, Nai-Feng Liu
Cellular Signalling.2022; 91: 110219. CrossRef - HIF-1 and NRF2; Key Molecules for Malignant Phenotypes of Pancreatic Cancer
Shin Hamada, Ryotaro Matsumoto, Atsushi Masamune
Cancers.2022; 14(2): 411. CrossRef - Pancreatic Stellate Cells and Metabolic Alteration: Physiology and Pathophysiology
Shin Hamada, Ryotaro Matsumoto, Atsushi Masamune
Frontiers in Physiology.2022;[Epub] CrossRef - Exosomal miR-140–3p and miR-143–3p from TGF-β1-treated pancreatic stellate cells target BCL2 mRNA to increase β-cell apoptosis
Xiangyun Zhu, Dechen Liu, Guoqing Li, Mengmeng Zhi, Ji Sun, Liang Qi, Jingbo Li, Stephen J. Pandol, Ling Li
Molecular and Cellular Endocrinology.2022; 551: 111653. CrossRef - Mitochondria oxidative stress mediated nicotine-promoted activation of pancreatic stellate cells by regulating mitochondrial dynamics
Yue Yuan, Zhiren Li, Miaomiao Li, Tong Jin, Xiaoyun Zhang, Xinjuan Liu, Jianyu Hao
Toxicology in Vitro.2022; 84: 105436. CrossRef - Antioxidant Mitoquinone Alleviates Chronic Pancreatitis via Anti-Fibrotic and Antioxidant Effects
Miaomiao Li, Yue Yuan, Xue Han, Xinjuan Liu, Weizhen Zhang, Jianyu Hao
Journal of Inflammation Research.2022; Volume 15: 4409. CrossRef - Diabetic Ferroptosis and Pancreatic Cancer: Foe or Friend?
Le Li, Xing-jia Yu, Lei Gao, Long Cheng, Bei Sun, Gang Wang
Antioxidants & Redox Signaling.2022; 37(16-18): 1206. CrossRef - Melatonin Induces Apoptosis and Modulates Cyclin Expression and MAPK Phosphorylation in Pancreatic Stellate Cells Subjected to Hypoxia
Matias Estaras, Manuel R. Gonzalez-Portillo, Miguel Fernandez-Bermejo, Jose M. Mateos, Daniel Vara, Gerardo Blanco-Fernandez, Diego Lopez-Guerra, Vicente Roncero, Gines M. Salido, Antonio González
International Journal of Molecular Sciences.2021; 22(11): 5555. CrossRef - Integrated pancreatic microcirculatory profiles of streptozotocin‐induced and insulin‐administrated type 1 diabetes mellitus
Yuan Li, Bingwei Li, Bing Wang, Mingming Liu, Xiaoyan Zhang, Ailing Li, Jian Zhang, Honggang Zhang, Ruijuan Xiu
Microcirculation.2021;[Epub] CrossRef - Pancreatic stellate cells - rising stars in pancreatic pathologies
P Hrabák, M Kalousová, T Krechler, T Zima
Physiological Research.2021; (S4): S597. CrossRef
- Effects of hypoxia in the diabetic corneal stroma microenvironment
- Basic Research
- The Role of CD36 in Type 2 Diabetes Mellitus: β-Cell Dysfunction and Beyond
- Jun Sung Moon, Udayakumar Karunakaran, Elumalai Suma, Seung Min Chung, Kyu Chang Won
- Diabetes Metab J. 2020;44(2):222-233. Published online April 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0053
- 7,521 View
- 169 Download
- 17 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Impaired β-cell function is the key pathophysiology of type 2 diabetes mellitus, and chronic exposure of nutrient excess could lead to this tragedy. For preserving β-cell function, it is essential to understand the cause and mechanisms about the progression of β-cells failure. Glucotoxicity, lipotoxicity, and glucolipotoxicity have been suggested to be a major cause of β-cell dysfunction for decades, but not yet fully understood. Fatty acid translocase cluster determinant 36 (CD36), which is part of the free fatty acid (FFA) transporter system, has been identified in several tissues such as muscle, liver, and insulin-producing cells. Several studies have reported that induction of CD36 increases uptake of FFA in several cells, suggesting the functional interplay between glucose and FFA in terms of insulin secretion and oxidative metabolism. However, we do not currently know the regulating mechanism and physiological role of CD36 on glucolipotoxicity in pancreatic β-cells. Also, the downstream and upstream targets of CD36 related signaling have not been defined. In the present review, we will focus on the expression and function of CD36 related signaling in the pancreatic β-cells in response to hyperglycemia and hyperlipidemia (ceramide) along with the clinical studies on the association between CD36 and metabolic disorders.
-
Citations
Citations to this article as recorded by- Nrf2 inhibition regulates intracellular lipid accumulation in mouse insulinoma cells and improves insulin secretory function
Alpana Mukhuty, Samanwita Mandal, Chandrani Fouzder, Snehasis Das, Dipanjan Chattopadhyay, Tanmay Majumdar, Rakesh Kundu
Molecular and Cellular Endocrinology.2024; 581: 112112. CrossRef - CD36 gene variant rs1761667(G/A) as a biomarker in obese type 2 diabetes mellitus cases
Ashwin Kumar Shukla, Amreen Shamsad, Atar Singh Kushwah, Shalini Singh, Kauser Usman, Monisha Banerjee
Egyptian Journal of Medical Human Genetics.2024;[Epub] CrossRef - CD36 regulates macrophage and endothelial cell activation and multinucleate giant cell formation in anti neutrophil cytoplasm antibody vasculitis
Xiang Zhang, Catherine King, Alexander Dowell, Paul Moss, Lorraine Harper, Dimitrios Chanouzas, Xiong-zhong Ruan, Alan David Salama
Clinical Immunology.2024; 260: 109914. CrossRef - The association of soluble cluster of differentiation 36 with metabolic diseases: A potential biomarker and therapeutic target
Yun Li, Yaxi Chen, Xiong Z. Ruan
Pediatric Discovery.2023;[Epub] CrossRef - The role of candidate transport proteins in β‐cell long‐chain fatty acid uptake: Where are we now?
Christina Clavelo‐Farrow, Patricia Thomas
Diabetic Medicine.2023;[Epub] CrossRef - SARS-CoV-2 in the pancreas and the impaired islet function in COVID-19 patients
Ningfei Ji, Mingshun Zhang, Liang Ren, Yunyun Wang, Bicheng Hu, Jie Xiang, Yingyun Gong, Chaojie Wu, Guoqiang Qu, Wenqiu Ding, Zhiqiang Yin, Shan Li, Zhengxia Wang, Lianzheng Zhou, Xueqin Chen, Yuan Ma, Jinhai Tang, Yun Liu, Liang Liu, Mao Huang
Emerging Microbes & Infections.2022; 11(1): 1115. CrossRef - Is imaging-based muscle quantity associated with risk of diabetes? A meta-analysis of cohort studies
Shanhu Qiu, Xue Cai, Yang Yuan, Bo Xie, Zilin Sun, Tongzhi Wu
Diabetes Research and Clinical Practice.2022; 189: 109939. CrossRef - Lipotoxicity in a Vicious Cycle of Pancreatic Beta Cell Exhaustion
Vladimir Grubelnik, Jan Zmazek, Matej Završnik, Marko Marhl
Biomedicines.2022; 10(7): 1627. CrossRef - Association of cluster determinant 36, scavenger receptor class B type 1, and major facilitator superfamily domain containing the 2a genetic polymorphism with serum lipid profile in aging population with type 2 diabetes mellitus
Xixiang Wang, Xiaojun Ma, Jingjing Xu, Yujie Guo, Shaobo Zhou, Huiyan Yu, Linhong Yuan
Frontiers in Nutrition.2022;[Epub] CrossRef - CD36-Fatty Acid-Mediated Metastasis via the Bidirectional Interactions of Cancer Cells and Macrophages
Noorzaileen Eileena Zaidi, Nur Aima Hafiza Shazali, Thean-Chor Leow, Mohd Azuraidi Osman, Kamariah Ibrahim, Wan-Hee Cheng, Kok-Song Lai, Nik Mohd Afizan Nik Abd Rahman
Cells.2022; 11(22): 3556. CrossRef - The Past and Present Lives of the Intraocular Transmembrane Protein CD36
Rucui Yang, Qingping Liu, Mingzhi Zhang
Cells.2022; 12(1): 171. CrossRef - Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus
Sachin Kumar, Tapan Behl, Monika Sachdeva, Aayush Sehgal, Shilpa Kumari, Arun Kumar, Gagandeep Kaur, Harlokesh Narayan Yadav, Simona Bungau
Life Sciences.2021; 264: 118661. CrossRef - Contribution of rs3211938 polymorphism at CD36 to glucose levels, oxidized low-density lipoproteins, insulin resistance, and body mass index in Mexican mestizos with type-2 diabetes from western Mexico
Beatriz Teresita Martín-Márquez, Flavio Sandoval-Garcia, Mónica Vazquez-Del Mercado, Erika-Aurora Martínez-García, Fernanda-Isadora Corona-Meraz, Ana-Lilia Fletes-Rayas, Soraya-Amalí Zavaleta-Muñiz
Nutrición Hospitalaria.2021;[Epub] CrossRef - Investigating the association of CD36 gene polymorphisms (rs1761667 and rs1527483) with T2DM and dyslipidemia: Statistical analysis, machine learning based prediction, and meta-analysis
Ma’mon M. Hatmal, Walhan Alshaer, Ismail S. Mahmoud, Mohammad A. I. Al-Hatamleh, Hamzeh J. Al-Ameer, Omar Abuyaman, Malek Zihlif, Rohimah Mohamud, Mais Darras, Mohammad Al Shhab, Rand Abu-Raideh, Hilweh Ismail, Ali Al-Hamadi, Ali Abdelhay, Kanhaiya Singh
PLOS ONE.2021; 16(10): e0257857. CrossRef - Misregulation of Wnt Signaling Pathways at the Plasma Membrane in Brain and Metabolic Diseases
Mustafa Karabicici, Yagmur Azbazdar, Evin Iscan, Gunes Ozhan
Membranes.2021; 11(11): 844. CrossRef
- Nrf2 inhibition regulates intracellular lipid accumulation in mouse insulinoma cells and improves insulin secretory function
- Clinical Diabetes & Therapeutics
- Three Months Monitored Metabolic Fitness Modulates Cardiovascular Risk Factors in Diabetic Patients
- Ilenia Cirilli, Sonia Silvestri, Fabio Marcheggiani, Fabiola Olivieri, Roberta Galeazzi, Roberto Antonicelli, Rina Recchioni, Fiorella Marcheselli, Tiziana Bacchetti, Luca Tiano, Patrick Orlando
- Diabetes Metab J. 2019;43(6):893-897. Published online June 27, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0254
- 3,904 View
- 46 Download
- 7 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Cardiovascular diseases represent the leading cause of death and moderate physical exercise is associated with a reduction in cardiovascular risk. The aim of the study was to evaluate the correlation between the amount of exercise recorded daily by a wearable gravitometer for 3 months and selected biochemical and clinical parameters. Nineteen sedentary type 2 diabetics were recruited and distributed into three homogenous groups, low, medium, and high exercise, according to the level of physical exercise monitored and expressed as MOVEs. Data showed an inverse correlation between MOVEs and oxidative stress indexes and a significant improvement in paraoxonase-1 activities and endothelial functionality. Decrease of visceral/total adipose tissue ratio, systolic blood pressure and a down-regulation of the inflammatory microRNA-146a in high exercise group were observed. Finally, a decrease of glycosylated hemoglobin and an up-regulation of the angiogenic microRNA-130a in medium exercise one was obtained. In this study, precise daily monitoring permitted to underline the importance of the amount of physical activity to counteract some cardiovascular risk factors persisting in diabetes. Finally, it identifies new microRNA biomarkers for future investigation on the same topic.
-
Citations
Citations to this article as recorded by- Emerging roles of microRNAs as diagnostics and potential therapeutic interest in type 2 diabetes mellitus
Dharmsheel Shrivastav, Desh Deepak Singh
World Journal of Clinical Cases.2024; 12(3): 525. CrossRef - Effects of Seven Weeks of Combined Physical Training on High-Density Lipoprotein Functionality in Overweight/Obese Subjects
Tiziana Bacchetti, Camilla Morresi, Gianna Ferretti, Anders Larsson, Torbjörn Åkerfeldt, Michael Svensson
Metabolites.2023; 13(10): 1068. CrossRef - Physical Exercise Protects Against Endothelial Dysfunction in Cardiovascular and Metabolic Diseases
Juan Gao, Xue Pan, Guoping Li, Emeli Chatterjee, Junjie Xiao
Journal of Cardiovascular Translational Research.2022; 15(3): 604. CrossRef - Effects of Exercise Training on the Paracrine Function of Circulating Angiogenic Cells
William S. Evans, Ryan M. Sapp, Katherine I. Kim, James M. Heilman, James Hagberg, Steven J. Prior
International Journal of Sports Medicine.2021; 42(12): 1047. CrossRef - Chronic and Transient Hyperglycemia Induces Changes in the Expression Patterns of IL6 and ADIPOQ Genes and Their Associated Epigenetic Modifications in Differentiating Human Visceral Adipocytes
Adam Wróblewski, Justyna Strycharz, Ewa Świderska, Aneta Balcerczyk, Janusz Szemraj, Józef Drzewoski, Agnieszka Śliwińska
International Journal of Molecular Sciences.2021; 22(13): 6964. CrossRef - The Potential Role of MicroRNA in Diabetic Cardiomyopathy
Jin Hwa Kim
Diabetes & Metabolism Journal.2020; 44(1): 54. CrossRef
- Emerging roles of microRNAs as diagnostics and potential therapeutic interest in type 2 diabetes mellitus
- Pathophysiology
- Metformin Ameliorates Lipotoxic β-Cell Dysfunction through a Concentration-Dependent Dual Mechanism of Action
- Hong Il Kim, Ji Seon Lee, Byung Kook Kwak, Won Min Hwang, Min Joo Kim, Young-Bum Kim, Sung Soo Chung, Kyong Soo Park
- Diabetes Metab J. 2019;43(6):854-866. Published online June 27, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0179
- 6,655 View
- 115 Download
- 14 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Chronic exposure to elevated levels of free fatty acids contributes to pancreatic β-cell dysfunction. Although it is well known that metformin induces cellular energy depletion and a concomitant activation of AMP-activated protein kinase (AMPK) through inhibition of the respiratory chain, previous studies have shown inconsistent results with regard to the action of metformin on pancreatic β-cells. We therefore examined the effects of metformin on pancreatic β-cells under lipotoxic stress.
Methods NIT-1 cells and mouse islets were exposed to palmitate and treated with 0.05 and 0.5 mM metformin. Cell viability, glucose-stimulated insulin secretion, cellular adenosine triphosphate, reactive oxygen species (ROS) levels and Rho kinase (ROCK) activities were measured. The phosphorylation of AMPK was evaluated by Western blot analysis and mRNA levels of endoplasmic reticulum (ER) stress markers and NADPH oxidase (NOX) were measured by real-time quantitative polymerase chain reaction analysis.
Results We found that metformin has protective effects on palmitate-induced β-cell dysfunction. Metformin at a concentration of 0.05 mM inhibits NOX and suppresses the palmitate-induced elevation of ER stress markers and ROS levels in a AMPK-independent manner, whereas 0.5 mM metformin inhibits ROCK activity and activates AMPK.
Conclusion This study suggests that the action of metformin on β-cell lipotoxicity was implemented by different molecular pathways depending on its concentration. Metformin at a usual therapeutic dose is supposed to alleviate lipotoxic β-cell dysfunction through inhibition of oxidative stress and ER stress.
-
Citations
Citations to this article as recorded by- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide
Si-min Zhou, Xin-ming Yao, Yi Cheng, Yu-jie Xing, Yue Sun, Qiang Hua, Shu-jun Wan, Xiang-jian Meng
Heliyon.2024; 10(2): e24432. CrossRef - Reduced Expression Level of Protein PhosphatasePPM1EServes to Maintain Insulin Secretion in Type 2 Diabetes
Sevda Gheibi, Luis Rodrigo Cataldo, Alexander Hamilton, Mi Huang, Sebastian Kalamajski, Malin Fex, Hindrik Mulder
Diabetes.2023; 72(4): 455. CrossRef - Metformin restores prohormone processing enzymes and normalizes aberrations in secretion of proinsulin and insulin in palmitate‐exposed human islets
Quan Wen, Azazul Islam Chowdhury, Banu Aydin, Mudhir Shekha, Rasmus Stenlid, Anders Forslund, Peter Bergsten
Diabetes, Obesity and Metabolism.2023; 25(12): 3757. CrossRef - Treatment of type 2 diabetes mellitus with stem cells and antidiabetic drugs: a dualistic and future-focused approach
Priyamvada Amol Arte, Kanchanlata Tungare, Mustansir Bhori, Renitta Jobby, Jyotirmoi Aich
Human Cell.2023; 37(1): 54. CrossRef - Metformin disrupts insulin secretion, causes proapoptotic and oxidative effects in rat pancreatic beta‐cells in vitro
Maíra M.R. Valle, Eloisa Aparecida Vilas‐Boas, Camila F. Lucena, Simone A. Teixeira, Marcelo N. Muscara, Angelo R. Carpinelli
Journal of Biochemical and Molecular Toxicology.2022;[Epub] CrossRef - Protection by metformin against severe Covid-19: An in-depth mechanistic analysis
Nicolas Wiernsperger, Abdallah Al-Salameh, Bertrand Cariou, Jean-Daniel Lalau
Diabetes & Metabolism.2022; 48(4): 101359. CrossRef - Insight Into Rho Kinase Isoforms in Obesity and Energy Homeostasis
Lei Wei, Jianjian Shi
Frontiers in Endocrinology.2022;[Epub] CrossRef - Overexpression of miR-297b-5p Promotes Metformin-Mediated Protection Against Stearic Acid-Induced Senescence by Targeting Igf1r
Qingrui Zhao, Shenghan Su, Yuqing Lin, Xuebei Li, Lingfeng Dan, Yunjin Zhang, Chunxiao Yang, Xiaohan Li, Yimeng Dong, Chenchen Geng, Changhao Sun, Xia Chu, Huimin Lu
SSRN Electronic Journal .2022;[Epub] CrossRef - Metformin Dysregulates the Unfolded Protein Response and the WNT/β-Catenin Pathway in Endometrial Cancer Cells through an AMPK-Independent Mechanism
Domenico Conza, Paola Mirra, Gaetano Calì, Luigi Insabato, Francesca Fiory, Francesco Beguinot, Luca Ulianich
Cells.2021; 10(5): 1067. CrossRef - NADPH Oxidase (NOX) Targeting in Diabetes: A Special Emphasis on Pancreatic β-Cell Dysfunction
Suma Elumalai, Udayakumar Karunakaran, Jun-Sung Moon, Kyu-Chang Won
Cells.2021; 10(7): 1573. CrossRef - Metformin use and cardiovascular outcomes in patients with diabetes and chronic kidney disease: a nationwide cohort study
Min Ho Kim, Hyung Jung Oh, Soon Hyo Kwon, Jin Seok Jeon, Hyunjin Noh, Dong Cheol Han, Hyoungnae Kim, Dong-Ryeol Ryu
Kidney Research and Clinical Practice.2021; 40(4): 660. CrossRef - Different Effects of Metformin and A769662 on Sodium Iodate-Induced Cytotoxicity in Retinal Pigment Epithelial Cells: Distinct Actions on Mitochondrial Fission and Respiration
Chi-Ming Chan, Ponarulselvam Sekar, Duen-Yi Huang, Shu-Hao Hsu, Wan-Wan Lin
Antioxidants.2020; 9(11): 1057. CrossRef - Metformin Reduces Lipotoxicity-Induced Meta-Inflammation in β-Cells through the Activation of GPR40-PLC-IP3 Pathway
Ximei Shen, Beibei Fan, Xin Hu, Liufen Luo, Yuanli Yan, Liyong Yang
Journal of Diabetes Research.2019; 2019: 1. CrossRef
- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide
- Islet Studies and Transplantation
-
- Myricetin Protects Against High Glucose-Induced β-Cell Apoptosis by Attenuating Endoplasmic Reticulum Stress via Inactivation of Cyclin-Dependent Kinase 5
- Udayakumar Karunakaran, Suma Elumalai, Jun Sung Moon, Jae-Han Jeon, Nam Doo Kim, Keun-Gyu Park, Kyu Chang Won, Jaechan Leem, In-Kyu Lee
- Diabetes Metab J. 2019;43(2):192-205. Published online January 16, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0052
- 4,913 View
- 106 Download
- 33 Web of Science
- 32 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Chronic hyperglycemia has deleterious effects on pancreatic β-cell function and turnover. Recent studies support the view that cyclin-dependent kinase 5 (CDK5) plays a role in β-cell failure under hyperglycemic conditions. However, little is known about how CDK5 impair β-cell function. Myricetin, a natural flavonoid, has therapeutic potential for the treatment of type 2 diabetes mellitus. In this study, we examined the effect of myricetin on high glucose (HG)-induced β-cell apoptosis and explored the relationship between myricetin and CDK5.
Methods To address this question, we subjected INS-1 cells and isolated rat islets to HG conditions (30 mM) in the presence or absence of myricetin. Docking studies were conducted to validate the interaction between myricetin and CDK5. Gene expression and protein levels of endoplasmic reticulum (ER) stress markers were measured by real-time reverse transcription polymerase chain reaction and Western blot analysis.
Results Activation of CDK5 in response to HG coupled with the induction of ER stress via the down regulation of sarcoendoplasmic reticulum calcium ATPase 2b (
SERCA2b ) gene expression and reduced the nuclear accumulation of pancreatic duodenal homeobox 1 (PDX1) leads to β-cell apoptosis. Docking study predicts that myricetin inhibit CDK5 activation by direct binding in the ATP-binding pocket. Myricetin counteracted the decrease in the levels of PDX1 and SERCA2b by HG. Moreover, myricetin attenuated HG-induced apoptosis in INS-1 cells and rat islets and reduce the mitochondrial dysfunction by decreasing reactive oxygen species production and mitochondrial membrane potential (Δψm) loss.Conclusion Myricetin protects the β-cells against HG-induced apoptosis by inhibiting ER stress, possibly through inactivation of CDK5 and consequent upregulation of PDX1 and SERCA2b.
-
Citations
Citations to this article as recorded by- Profiling of secondary metabolite and evaluation of anti-diabetic potency of Crotalaria quinquefolia (L): In-vitro, in-vivo, and in-silico approaches
Nazmun Nahar, Md. Nazmul Hasan Zilani, Partha Biswas, Md. Morsaline Billah, Shabana Bibi, Norah A. Albekairi, Abdulrahman Alshammari, Md. Nazmul Hasan
Saudi Pharmaceutical Journal.2024; 32(1): 101887. CrossRef - Mitochondrial aldehyde dehydrogenase-2 coordinates the hydrogen sulfide - AMPK axis to attenuate high glucose-induced pancreatic β-cell dysfunction by glutathione antioxidant system
Udayakumar Karunakaran, Suma Elumalai, Seung Min Chung, Kathrin Maedler, Kyu Chang Won, Jun Sung Moon
Redox Biology.2024; 69: 102994. CrossRef - Network-based identification and mechanism exploration of active ingredients against Alzheimer’s disease via targeting endoplasmic reticulum stress from traditional chinese medicine
Zhao Dai, Tian Hu, Junwen Wei, Xue Wang, Chuipu Cai, Yong Gu, Yunhui Hu, Wenjia Wang, Qihui Wu, Jiansong Fang
Computational and Structural Biotechnology Journal.2024; 23: 506. CrossRef - Myricetin as a Promising Flavonoid with Multitargeted Biological Activity
A.S. Chiriapkin
Juvenis Scientia.2024; 10(1): 5. CrossRef - Naturally occurring small molecules with dual effect upon inflammatory signaling pathways and endoplasmic reticulum stress response
Daniela Correia da Silva, Patrícia Valentão, David M. Pereira
Journal of Physiology and Biochemistry.2024;[Epub] CrossRef - Omnifarious fruit polyphenols: an omnipotent strategy to prevent and intervene diabetes and related complication?
Yao Chen, Xuejiao Qie, Wei Quan, Maomao Zeng, Fang Qin, Jie Chen, Benu Adhikari, Zhiyong He
Critical Reviews in Food Science and Nutrition.2023; 63(20): 4288. CrossRef - Regulation of reactive oxygen species by phytochemicals for the management of cancer and diabetes
Heui Min Lim, See-Hyoung Park
Critical Reviews in Food Science and Nutrition.2023; 63(22): 5911. CrossRef - Bioactive compounds from Polygonatum genus as anti-diabetic agents with future perspectives
Yan Shi, Dun Si, Donghong Chen, Xinfeng Zhang, Zhigang Han, Qiang Yu, Jingjing Liu, Jinping Si
Food Chemistry.2023; 408: 135183. CrossRef - Venom Peptides, Polyphenols and Alkaloids: Are They the Next Antidiabetics That Will Preserve β-Cell Mass and Function in Type 2 Diabetes?
Michele Lodato, Valérie Plaisance, Valérie Pawlowski, Maxime Kwapich, Alexandre Barras, Emeline Buissart, Stéphane Dalle, Sabine Szunerits, Jérôme Vicogne, Rabah Boukherroub, Amar Abderrahmani
Cells.2023; 12(6): 940. CrossRef - TFP5 attenuates cyclin‐dependent kinase 5‐mediated islet β‐cell damage in diabetes
Shunyao Liu, Bo Li, Danna Ma, Yuejia Tao, Jiang Song, Li Bao, Guoqing Zhang, Hongyan Luo, Shilu Cao, Jing E, Yali Zheng
Chemical Biology & Drug Design.2023; 102(1): 76. CrossRef - Antiviral and Possible Prophylactic Significance of Myricetin for COVID-19
Pawan K. Agrawal, Chandan Agrawal, Gerald Blunden
Natural Product Communications.2023; 18(4): 1934578X2311662. CrossRef - In Vitro and In Silico Protocols for the Assessment of Anti-Tick Compounds from Pinus roxburghii against Rhipicephalus (Boophilus) microplus Ticks
Sana Ayub, Nosheen Malak, Raquel Cossío-Bayúgar, Nasreen Nasreen, Afshan Khan, Sadaf Niaz, Adil Khan, Abdallah D. Alanazi, Mourad Ben Said
Animals.2023; 13(8): 1388. CrossRef - Protective Effect of Myricetin Against Experimentally Induced Torsion in Rats
M. Tatar, Z. Polat, J. Öner, H. Öner
Biology Bulletin.2023; 50(6): 1338. CrossRef - The pharmacological mechanism of Abelmoschus manihot in the treatment of chronic kidney disease
Cuiting Wei, Chao Wang, Run Li, Yunfeng Bai, Xue Wang, Qingyun Fang, Xiangmei Chen, Ping Li
Heliyon.2023; 9(11): e22017. CrossRef - Natural Polyphenols as SERCA Activators: Role in the Endoplasmic Reticulum Stress-Related Diseases
Jana Viskupicova, Petronela Rezbarikova
Molecules.2022; 27(16): 5095. CrossRef - Research progress on the mechanism of beta-cell apoptosis in type 2 diabetes mellitus
SuFang You, JingYi Zheng, YuPing Chen, HuiBin Huang
Frontiers in Endocrinology.2022;[Epub] CrossRef - Myricetin inhibits pseudorabies virus infection through direct inactivation and activating host antiviral defense
Huaiyue Hu, Zhiqiang Hu, Yingying Zhang, Hongping Wan, Zhongqiong Yin, Lixia Li, Xiaoxia Liang, Xinghong Zhao, Lizi Yin, Gang Ye, Yuan-Feng Zou, Huaqiao Tang, Renyong Jia, Yaqin Chen, Hao Zhou, Xu Song
Frontiers in Microbiology.2022;[Epub] CrossRef - Effects of myricetin against cadmium-induced neurotoxicity in PC12 cells
Azadeh Aminzadeh, Ayda Salarinejad
Toxicology Research.2021; 10(1): 84. CrossRef - Pioglitazone-induced AMPK-Glutaminase-1 prevents high glucose-induced pancreatic β-cell dysfunction by glutathione antioxidant system
Udayakumar Karunakaran, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Redox Biology.2021; 45: 102029. CrossRef - Chlorogenic acid and β-glucan from highland barley grain ameliorate β-cell dysfunction via inhibiting apoptosis and improving cell proliferation
Ze-Hua Liu, Bo Li
Food & Function.2021; 12(20): 10040. CrossRef - The cyclin dependent kinase inhibitor Roscovitine prevents diet-induced metabolic disruption in obese mice
Nabil Rabhi, Kathleen Desevin, Briana Noel Cortez, Ryan Hekman, Jean Z. Lin, Andrew Emili, Stephen R. Farmer
Scientific Reports.2021;[Epub] CrossRef - AdipoRon promotes diabetic fracture repair through endochondral ossification-based bone repair by enhancing survival and differentiation of chondrocytes
Zhongyi Wang, Jinxin Tang, Ying Li, Yu Wang, Yanyang Guo, Qisheng Tu, Jake Chen, Chen Wang
Experimental Cell Research.2020; 387(2): 111757. CrossRef - A kinase of many talents: non-neuronal functions of CDK5 in development and disease
Samanta Sharma, Piotr Sicinski
Open Biology.2020; 10(1): 190287. CrossRef - Mitochondrial dysfunction in the fetoplacental unit in gestational diabetes mellitus
Luis Sobrevia, Paola Valero, Adriana Grismaldo, Roberto Villalobos-Labra, Fabián Pardo, Mario Subiabre, Gael Armstrong, Fernando Toledo, Sofía Vega, Marcelo Cornejo, Gonzalo Fuentes, Reinaldo Marín
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2020; 1866(12): 165948. CrossRef - Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications
Yasaman Taheri, Hafiz Ansar Rasul Suleria, Natália Martins, Oksana Sytar, Ahmet Beyatli, Balakyz Yeskaliyeva, Gulnaz Seitimova, Bahare Salehi, Prabhakar Semwal, Sakshi Painuli, Anuj Kumar, Elena Azzini, Miquel Martorell, William N. Setzer, Alfred Maroyi,
BMC Complementary Medicine and Therapies.2020;[Epub] CrossRef - Current Pharmacological Trends on Myricetin
Gudiya Gupta, Mohd Aftab Siddiqui, Mohd Muazzam Khan, Mohd Ajmal, Rabiya Ahsan, Md Azizur Rahaman, Md Afroz Ahmad, Md Arshad, Mohammad Khushtar
Drug Research.2020;[Epub] CrossRef - Silencing cyclophilin A improves insulin secretion, reduces cell apoptosis, and alleviates inflammation as well as oxidant stress in high glucose-induced pancreatic β-cells via MAPK/NF-kb signaling pathway
Tangying Li, Huibiao Quan, Huachuan Zhang, Leweihua Lin, Qianying Ou, Kaining Chen
Bioengineered.2020; 11(1): 1047. CrossRef - Endoplasmic reticulum stress contributes to NMDA-induced pancreatic β-cell dysfunction in a CHOP-dependent manner
Xiao-Ting Huang, Wei Liu, Yong Zhou, Mei Sun, Chen-Chen Sun, Chen-Yu Zhang, Si-Yuan Tang
Life Sciences.2019; 232: 116612. CrossRef - Dapagliflozin rescues endoplasmic reticulum stress-mediated cell death
Ryo Shibusawa, Eijiro Yamada, Shuichi Okada, Yasuyo Nakajima, Claire C. Bastie, Akito Maeshima, Kyoichi Kaira, Masanobu Yamada
Scientific Reports.2019;[Epub] CrossRef - Myricetin prevents thapsigargin-induced CDK5-P66Shc signalosome mediated pancreatic β-cell dysfunction
Udayakumar Karunakaran, Ji Eun Lee, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Free Radical Biology and Medicine.2019; 141: 59. CrossRef - CDK5: Key Regulator of Apoptosis and Cell Survival
Rabih Roufayel, Nimer Murshid
Biomedicines.2019; 7(4): 88. CrossRef - Oral DhHP-6 for the Treatment of Type 2 Diabetes Mellitus
Kai Wang, Yu Su, Yuting Liang, Yanhui Song, Liping Wang
International Journal of Molecular Sciences.2019; 20(6): 1517. CrossRef
- Profiling of secondary metabolite and evaluation of anti-diabetic potency of Crotalaria quinquefolia (L): In-vitro, in-vivo, and in-silico approaches
- Pathophysiology
- Nuclear Receptors Resolve Endoplasmic Reticulum Stress to Improve Hepatic Insulin Resistance
- Jae Man Lee
- Diabetes Metab J. 2017;41(1):10-19. Published online February 16, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.1.10
- 4,592 View
- 94 Download
- 13 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Chronic endoplasmic reticulum (ER) stress culminating in proteotoxicity contributes to the development of insulin resistance and progression to type 2 diabetes mellitus. Pharmacologic interventions targeting several different nuclear receptors have emerged as potential treatments for insulin resistance. The mechanistic basis for these antidiabetic effects has primarily been attributed to multiple metabolic and inflammatory functions. Here we review recent advances in our understanding of the association of ER stress with insulin resistance and the role of nuclear receptors in promoting ER stress resolution and improving insulin resistance in the liver.
-
Citations
Citations to this article as recorded by- Duality of Nrf2 in iron-overload cardiomyopathy
Enrica Federti, Francesca Vinchi, Iana Iatcenko, Alessandra Ghigo, Alessandro Matte, Serge Cedrick Mbiandjeu Toya, Angela Siciliano, Deborah Chiabrando, Emanuela Tolosano, Steven Zebulon Vance, Veronica Riccardi, Immacolata Andolfo, Manuela Iezzi, Alessia
Haematologica.2023; 108(5): 1335. CrossRef - Endoplasmic Reticulum Stress and Its Impact on Adipogenesis: Molecular Mechanisms Implicated
Gyuhui Kim, Jiyoon Lee, Joohun Ha, Insug Kang, Wonchae Choe
Nutrients.2023; 15(24): 5082. CrossRef - Qingluotongbi formula regulates the LXRα-ERS-SREBP-1c pathway in hepatocytes to alleviate the liver injury caused by Tripterygium wilfordii Hook. f.
Zhichao Yu, Zhe Feng, Ling Fu, Jing Wang, Changqing Li, Huaxu Zhu, Tong Xie, Jie Zhou, Lingling Zhou, Xueping Zhou
Journal of Ethnopharmacology.2022; 287: 114952. CrossRef - Nuclear‐mitochondrial crosstalk: On the role of the nuclear receptor liver receptor homolog‐1 (NR5A2) in the regulation of mitochondrial metabolism, cell survival, and cancer
Svenja Michalek, Thomas Brunner
IUBMB Life.2021; 73(3): 592. CrossRef - NGBR is required to ameliorate type 2 diabetes in mice by enhancing insulin sensitivity
Yi Chen, Wenquan Hu, Qi Li, Shiwei Zhao, Dan Zhao, Shuang Zhang, Zhuo Wei, Xiaoxiao Yang, Yuanli Chen, Xiaoju Li, Chenzhong Liao, Jihong Han, Qing Robert Miao, Yajun Duan
Journal of Biological Chemistry.2021; 296: 100624. CrossRef - Impaired ferritinophagy flux induced by high fat diet mediates hepatic insulin resistance via endoplasmic reticulum stress
Chunjie Jiang, Shanshan Zhang, Dan Li, Li Chen, Ying Zhao, Guibin Mei, Jingjing Liu, Yuhan Tang, Chao Gao, Ping Yao
Food and Chemical Toxicology.2020; 140: 111329. CrossRef - Dipeptidyl peptidase-4 inhibitor protects against non-alcoholic steatohepatitis in mice by targeting TRAIL receptor-mediated lipoapoptosis via modulating hepatic dipeptidyl peptidase-4 expression
Minyoung Lee, Eugene Shin, Jaehyun Bae, Yongin Cho, Ji-Yeon Lee, Yong-ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha
Scientific Reports.2020;[Epub] CrossRef - Use of fenofibrate on cardiovascular outcomes in statin users with metabolic syndrome: propensity matched cohort study
Nam Hoon Kim, Ki Hoon Han, Jimi Choi, Juneyoung Lee, Sin Gon Kim
BMJ.2019; : l5125. CrossRef - Inhibition of the Low Molecular Weight Protein Tyrosine Phosphatase (LMPTP) as a Potential Therapeutic Strategy for Hepatic Progenitor Cells Lipotoxicity—Short Communication
Michalina Alicka, Katarzyna Kornicka-Garbowska, Michael Roecken, Krzysztof Marycz
International Journal of Molecular Sciences.2019; 20(23): 5873. CrossRef - Myricetin prevents thapsigargin-induced CDK5-P66Shc signalosome mediated pancreatic β-cell dysfunction
Udayakumar Karunakaran, Ji Eun Lee, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Free Radical Biology and Medicine.2019; 141: 59. CrossRef - Spontaneous ketonuria and risk of incident diabetes: a 12 year prospective study
Gyuri Kim, Sang-Guk Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Ele Ferrannini, Yong-ho Lee, Nam H. Cho
Diabetologia.2019; 62(5): 779. CrossRef - CCAAT/enhancer binding protein homologous protein knockdown alleviates hypoxia-induced myocardial injury in rat cardiomyocytes exposed to high glucose
Wenqi Yang, Fang Wu, Ting Luo, Yuelan Zhang
Experimental and Therapeutic Medicine.2018;[Epub] CrossRef - Association of changes in ER stress-mediated signaling pathway with lead-induced insulin resistance and apoptosis in rats and their prevention by A-type dimeric epigallocatechin-3-gallate
Chan-Min Liu, Jie-Qiong Ma, Jian-Mei Sun, Zhao-Jun Feng, Chao Cheng, Wei Yang, Hong Jiang
Food and Chemical Toxicology.2017; 110: 325. CrossRef
- Duality of Nrf2 in iron-overload cardiomyopathy
- Serum Ceruloplasmin Level as a Predictor for the Progression of Diabetic Nephropathy in Korean Men with Type 2 Diabetes Mellitus
- Min Jung Lee, Chang Hee Jung, Yu Mi Kang, Jung Eun Jang, Jaechan Leem, Joong-Yeol Park, Woo Je Lee
- Diabetes Metab J. 2015;39(3):230-239. Published online April 22, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.3.230
- 4,415 View
- 46 Download
- 22 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Oxidative stress is known to be associated with progression of diabetic kidney disease. Ceruloplasmin acts as a pro-oxidant under conditions of severe oxidative stress. Thus, we conducted a longitudinal observational study to evaluate whether the serum ceruloplasmin level is a predictive biomarker for progression of diabetic nephropathy.
Methods A total of 643 Korean men with type 2 diabetes mellitus were enrolled. Serum ceruloplasmin was measured using a nephelometric method. Progression of diabetic nephropathy was defined as transition in albuminuria class (i.e., normoalbuminuria to microalbuminuria, microalbuminuria to macroalbuminuria, or normoalbuminuria to macroalbuminuria) and/or a greater than 2-fold increase of serum creatinine at follow-up compared with the baseline value.
Results During the follow-up period (median, 2.7 years; range, 0.3 to 4.4 years), 49 of 643 patients (7.6%) showed the progression of diabetic nephropathy and three patients (0.5%) developed end-stage renal disease. Baseline ceruloplasmin levels were higher in the progressors than in the nonprogressors (262.6±40.9 mg/L vs. 233.3±37.8 mg/L,
P <0.001). Kaplan-Meier analysis showed a significantly higher incidence of nephropathy progression according to ceruloplasmin tertile (log-rank test,P <0.001). The hazard ratio (HR) for progression of diabetic nephropathy was significantly higher in the highest ceruloplasmin tertile category compared with the lowest ceruloplasmin tertile category, even after adjusting for confounding variables (HR, 3.32; 95% confidence interval, 1.28 to 8.61;P =0.003).Conclusion Baseline serum ceruloplasmin is an independent predictive factor for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus.
-
Citations
Citations to this article as recorded by- In-depth urinary and exosome proteome profiling analysis identifies novel biomarkers for diabetic kidney disease
Shichun Du, Linhui Zhai, Shu Ye, Le Wang, Muyin Liu, Minjia Tan
Science China Life Sciences.2023; 66(11): 2587. CrossRef - Serum Level of Ceruloplasmin, Angiotensin-Converting Enzyme and Transferrin as Markers of Severity in SARS-CoV-2 Infection in Patients with Type 2 Diabetes
Patricia-Andrada Reștea, Ștefan Țigan, Laura Grațiela Vicaș, Luminița Fritea, Eleonora Marian, Tunde Jurca, Annamaria Pallag, Iulius Liviu Mureșan, Corina Moisa, Otilia Micle, Mariana Eugenia Mureșan
Microbiology Research.2023; 14(4): 1670. CrossRef - The nephropathy of sickle cell trait and sickle cell disease
Kenneth I. Ataga, Santosh L. Saraf, Vimal K. Derebail
Nature Reviews Nephrology.2022; 18(6): 361. CrossRef - Integrated Analysis of Single-Cell RNA-seq and Bulk RNA-seq in the Identification of a Novel ceRNA Network and Key Biomarkers in Diabetic Kidney Disease
Yuejun Wang, Mingming Zhao, Yu Zhang
International Journal of General Medicine.2022; Volume 15: 1985. CrossRef - Molecular Functions of Ceruloplasmin in Metabolic Disease Pathology
Zhidong Liu, Miao Wang, Chunbo Zhang, Shigao Zhou, Guang Ji
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2022; Volume 15: 695. CrossRef - A correlative study of copper, ceruloplasmin, iron, total iron binding capacity and total antioxidant capacity in diabetic nephropathy
Ramlingareddy, Shivashankara A Ramachandrayya, Jeena Jacob, Malathi Mala
Biomedicine.2022; 42(3): 469. CrossRef - Novel biomarkers for prognosticating diabetic kidney disease progression
Shilna Muttickal Swaminathan, Indu Ramachandra Rao, Srinivas Vinayak Shenoy, Attur Ravindra Prabhu, Pooja Basthi Mohan, Dharshan Rangaswamy, Mohan V Bhojaraja, Shivashankara Kaniyoor Nagri, Shankar Prasad Nagaraju
International Urology and Nephrology.2022; 55(4): 913. CrossRef - Evaluation of Serum Ceruloplasmin Levels as a Biomarker for Oxidative Stress in Patients With Diabetic Retinopathy
Gurunadh Satyanarayana, Narendra Keisham, Hitender S Batra, Subrahmanya Murti V, Mansur Khan, Sandeep Gupta, Vikram Mahindra
Cureus.2021;[Epub] CrossRef - Prospection of plasma proteins as biomarkers for diabetes mellitus monitoring
Liliane de Paula Silva, Fabiane Gomes de Moraes Rego, Geraldo Picheth, Marcelo Müller-Santos, Dayane Alberton
Journal of Diabetes & Metabolic Disorders.2021; 20(1): 611. CrossRef - Risk assessment for foot ulcers among Tunisian subjects with diabetes: a cross sectional outpatient study
B. Zantour, S. Bouchareb, Z. El Ati, F. Boubaker, W. Alaya, W. Kossomtini, M. H. Sfar
BMC Endocrine Disorders.2020;[Epub] CrossRef - Metalloproteins and apolipoprotein C: candidate plasma biomarkers of T2DM screened by comparative proteomics and lipidomics in ZDF rats
Shuai Wang, Zhiyuan Lu, Yuxin Wang, Tianran Zhang, Xiaodong He
Nutrition & Metabolism.2020;[Epub] CrossRef - Emerging vistas on electrochemical detection of diabetic retinopathy biomarkers
K.S. Shalini Devi, Madhurantakam Sasya, Uma Maheswari Krishnan
TrAC Trends in Analytical Chemistry.2020; 125: 115838. CrossRef - Oxidative stress in peritoneal dialysis patients: Association with the dialysis adequacy and technique survival
Natalia Stepanova, Lesya Korol, Olena Burdeyna
Indian Journal of Nephrology.2019; 29(5): 309. CrossRef - Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management
Veena Vijayakumar, Sushanta K. Samal, Smita Mohanty, Sanjay K. Nayak
International Journal of Biological Macromolecules.2019; 122: 137. CrossRef - Rat Böbrek Dokusunda Kurşunun Neden Olduğu Oksidatif Strese Karşı Kitosanın Koruyucu etkisi
Ugur ÖZDEK, Hasan TOZ, Ahmet Ufuk KÖMÜROĞLU, Leyla MİS, Zübeyir HUYUT, Yeter DEĞER
Van Veterinary Journal.2019; 30(3): 187. CrossRef - Changes in Trace Elements During Early Stages of Chronic Kidney Disease in Type 2 Diabetic Patients
Ching-Chiang Lin, Ching-Tang Shih, Chien-Hung Lee, Yeou-Lih Huang
Biological Trace Element Research.2018; 186(2): 330. CrossRef - The Divalent Elements Changes in Early Stages of Chronic Kidney Disease
Wan-Ju Kung, Ching-Tang Shih, Chien-Hung Lee, Ching-Chiang Lin
Biological Trace Element Research.2018; 185(1): 30. CrossRef - Long-term expression of glomerular genes in diabetic nephropathy
Dominik Chittka, Bernhard Banas, Laura Lennartz, Franz Josef Putz, Kathrin Eidenschink, Sebastian Beck, Thomas Stempfl, Christoph Moehle, Simone Reichelt-Wurm, Miriam C Banas
Nephrology Dialysis Transplantation.2018;[Epub] CrossRef - Incidence of chronic kidney disease among people with diabetes: a systematic review of observational studies
D. N. Koye, J. E. Shaw, C. M. Reid, R. C. Atkins, A. T. Reutens, D. J. Magliano
Diabetic Medicine.2017; 34(7): 887. CrossRef - Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis
Pengzi Zhang, Jing Lu, Yali Jing, Sunyinyan Tang, Dalong Zhu, Yan Bi
Annals of Medicine.2017; 49(2): 106. CrossRef - Biomarkers of diabetic nephropathy: A 2017 update
Nektaria Papadopoulou-Marketou, Christina Kanaka-Gantenbein, Nikolaos Marketos, George P. Chrousos, Ioannis Papassotiriou
Critical Reviews in Clinical Laboratory Sciences.2017; 54(5): 326. CrossRef - Serum Vascular Adhesion Protein-1 Predicts End-Stage Renal Disease in Patients with Type 2 Diabetes
Hung-Yuan Li, Hung-An Lin, Feng-Jung Nien, Vin-Cent Wu, Yi-Der Jiang, Tien-Jyun Chang, Hsien-Li Kao, Mao-Shin Lin, Jung-Nan Wei, Cheng-Hsin Lin, Shyang-Rong Shih, Chi-Sheng Hung, Lee-Ming Chuang, Emmanuel A Burdmann
PLOS ONE.2016; 11(2): e0147981. CrossRef
- In-depth urinary and exosome proteome profiling analysis identifies novel biomarkers for diabetic kidney disease
- Diabetic Cardiomyopathy and Its Prevention by Nrf2: Current Status
- Jing Chen, Zhiguo Zhang, Lu Cai
- Diabetes Metab J. 2014;38(5):337-345. Published online October 17, 2014
- DOI: https://doi.org/10.4093/dmj.2014.38.5.337
- 5,082 View
- 55 Download
- 76 Web of Science
- 75 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Diabetic cardiomyopathy (DCM), as one of the major cardiac complications in diabetic patients, is known to related with oxidative stress that is due to a severe imbalance between reactive oxygen species (ROS) and/or reactive nitrogen species (RNS) generation and their clearance by antioxidant defense systems. Transcription factor nuclear factor NF-E2-related factor 2 (Nrf2) plays an important role in maintaining the oxidative homeostasis by regulating multiple downstream antioxidants. Diabetes may up-regulate several antioxidants in the heart as a compensative mechanism at early stage, but at late stage, diabetes not only generates extra ROS and/or RNS but also impairs antioxidant capacity in the heart, including Nrf2. In an early study, we have established that Nrf2 protect the cardiac cells and heart from high level of glucose
in vitro and hyperglycemiain vivo , and in the following study demonstrated the significant down-regulation of cardiac Nrf2 expression in diabetic animals and patients. Using Nrf2-KO mice or Nrf2 inducers, blooming evidence has indicated the important protection by Nrf2 from cardiac pathogenesis in the diabetes. Therefore, this brief review summarizes the status of studies on Nrf2's role in preventing DCM and even other complications, the need for new and safe Nrf2 inducer screening and the precaution for the undesirable side of Nrf2 under certain conditions.-
Citations
Citations to this article as recorded by- Natural Products and Health
Joanna Bartkowiak-Wieczorek, Edyta Mądry
Nutrients.2024; 16(3): 415. CrossRef - Nrf2 prevents diabetic cardiomyopathy via antioxidant effect and normalization of glucose and lipid metabolism in the heart
Ge Yang, Qihe Zhang, Chao Dong, Guowen Hou, Jinjie Li, Xin Jiang, Ying Xin
Journal of Cellular Physiology.2024;[Epub] CrossRef - 3,4′,5-Trimethoxy-trans-stilbene ameliorates hepatic insulin resistance and oxidative stress in diabetic obese mice through insulin and Nrf2 signaling pathways
Yi Tan, Chunxiu Zhou, Lingchao Miao, Xutao Zhang, Haroon Khan, Baojun Xu, Wai San Cheang
Food & Function.2024; 15(6): 2996. CrossRef - Natural compounds protect against the pathogenesis of osteoarthritis by mediating the NRF2/ARE signaling
Zhenyu Wu, Zhouxin Yang, Luying Liu, Yong Xiao
Frontiers in Pharmacology.2023;[Epub] CrossRef - High fat diet exacerbates long-term metabolic, neuropathological, and behavioral derangements in an experimental mouse model of traumatic brain injury
Stanley Ibeh, Nour-Mounira Z. Bakkar, Fatima Ahmad, Judith Nwaiwu, Chloe Barsa, Sarine Mekhjian, Mohammad Amine Reslan, Ali H. Eid, Hayat Harati, Sanaa Nabha, Yehia Mechref, Ahmed F. El-Yazbi, Firas Kobeissy
Life Sciences.2023; 314: 121316. CrossRef - The Protective Effect of 11-Keto-β-Boswellic Acid against Diabetic Cardiomyopathy in Rats Entails Activation of AMPK
Jozaa Z. AlTamimi, Nora A. AlFaris, Ghedeir M. Alshammari, Reham I. Alagal, Dalal H. Aljabryn, Mohammed Abdo Yahya
Nutrients.2023; 15(7): 1660. CrossRef - Caffeic Acid Phenethyl Ester and Caffeamide Derivatives Suppress Oral Squamous Cell Carcinoma Cells
Yin-Hwa Shih, Chieh-Chieh Chen, Yueh-Hsiung Kuo, Lih-Jyh Fuh, Wan-Chen Lan, Tong-Hong Wang, Kuo-Chou Chiu, Thanh-Hien Vu Nguyen, Shih-Min Hsia, Tzong-Ming Shieh
International Journal of Molecular Sciences.2023; 24(12): 9819. CrossRef - The Role of Asiatic Acid in Preventing Dental Pulp Inflammation: An in-vivo Study
Arlina Nurhapsari, Risya Cilmiaty, Adi Prayitno, Bambang Purwanto, Soetrisno Soetrisno
Clinical, Cosmetic and Investigational Dentistry.2023; Volume 15: 109. CrossRef - Dexmedetomidine ameliorates diabetic cardiomyopathy by inhibiting ferroptosis through the Nrf2/GPX4 pathway
Fan Li, Zhenfei Hu, Yidan Huang, Haiting Zhan
Journal of Cardiothoracic Surgery.2023;[Epub] CrossRef - Metformin-mediated epigenetic modifications in diabetes and associated conditions: Biological and clinical relevance
Roberta Giordo, Anna Maria Posadino, Arduino Aleksander Mangoni, Gianfranco Pintus
Biochemical Pharmacology.2023; 215: 115732. CrossRef - Transient receptor potential vanilloid type 1: cardioprotective effects in diabetic models
Jiaqi Bao, Zhicheng Gao, Yilan Hu, Lifang Ye, Lihong Wang
Channels.2023;[Epub] CrossRef - Beyond Hepatoprotection—The Cardioprotective Effects of Bicyclol in Diabetes
Arun Samidurai, Rakesh C. Kukreja
Cardiovascular Drugs and Therapy.2023;[Epub] CrossRef - Sinapic acid ameliorates cardiac dysfunction and cardiomyopathy by modulating NF-κB and Nrf2/HO-1 signaling pathways in streptozocin induced diabetic rats
Mohammad Raish, Ajaz Ahmad, Yousef A. Bin Jardan, Mudassar Shahid, Khalid M. Alkharfy, Abdul Ahad, Mushtaq Ahmad Ansari, Ibrahim Abdelsalam Abdelrahman, Fahad I. Al-Jenoobi
Biomedicine & Pharmacotherapy.2022; 145: 112412. CrossRef - Recent Advances in KEAP1/NRF2-Targeting Strategies by Phytochemical Antioxidants, Nanoparticles, and Biocompatible Scaffolds for the Treatment of Diabetic Cardiovascular Complications
Andrea Rampin, Michele Carrabba, Martina Mutoli, Charlotte L. Eman, Gianluca Testa, Paolo Madeddu, Gaia Spinetti
Antioxidants & Redox Signaling.2022; 36(10-12): 707. CrossRef - Exercise and Urtica Dioica extract ameliorate mitochondrial function and the expression of cardiac muscle Nuclear Respiratory Factor 2 and Peroxisome proliferator-activated receptor Gamma Coactivator 1-alpha in STZ-induced diabetic rats
Seyyedeh Masoumeh Seyydi, Asghar Tofighi, Masoud Rahmati, Javad Tolouei Azar
Gene.2022; 822: 146351. CrossRef - 3,3′,4,5′-Tetramethoxy-trans-stilbene Improves Insulin Resistance by Activating the IRS/PI3K/Akt Pathway and Inhibiting Oxidative Stress
Yi Tan, Lingchao Miao, Jianbo Xiao, Wai San Cheang
Current Issues in Molecular Biology.2022; 44(5): 2175. CrossRef - The Beneficial Effects of Chinese Herbal Monomers on Ameliorating Diabetic Cardiomyopathy via Nrf2 Signaling
Yiwei Gao, Wu Liu, Xin Su, Xinyi Li, Fangning Yu, Ning Zhang, Yuli Huang
Oxidative Medicine and Cellular Longevity.2022; 2022: 1. CrossRef - Cardioprotective Effects and in-silico Antioxidant Mechanism of L-Ergothioneine in Experimental Type-2 Diabetic Rats
Ayobami Dare, Ahmed A Elrashedy, Mahendra L. Channa, Anand Nadar
Cardiovascular & Hematological Agents in Medicinal Chemistry.2022; 20(2): 133. CrossRef - Molecular Mechanisms of Ferroptosis and Relevance to Cardiovascular Disease
Lai-Hua Xie, Nadezhda Fefelova, Sri Harika Pamarthi, Judith K. Gwathmey
Cells.2022; 11(17): 2726. CrossRef - Cardiac adaptation and cardioprotection against arrhythmias and ischemia-reperfusion injury in mammalian hibernators
Lai-Hua Xie, Judith K. Gwathmey, Zhenghang Zhao
Pflügers Archiv - European Journal of Physiology.2021; 473(3): 407. CrossRef - Cardioprotective Effect of Glycyrrhizin on Myocardial Remodeling in Diabetic Rats
Vikram Thakur, Narah Alcoreza, Monica Delgado, Binata Joddar, Munmun Chattopadhyay
Biomolecules.2021; 11(4): 569. CrossRef - Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy
Nikole J. Byrne, Namakkal S. Rajasekaran, E Dale Abel, Heiko Bugger
Free Radical Biology and Medicine.2021; 169: 317. CrossRef - Punicalagin Protects Against Diabetic Cardiomyopathy by Promoting Opa1-Mediated Mitochondrial Fusion via Regulating PTP1B-Stat3 Pathway
Feng Fu, Chaoyang Liu, Rui Shi, Man Li, Min Zhang, Yanyan Du, Qiaojuan Wang, Jun Li, Guoen Wang, Jianming Pei, Mingge Ding
Antioxidants & Redox Signaling.2021; 35(8): 618. CrossRef - Elevated type I interferon responses potentiate metabolic dysfunction, inflammation, and accelerated aging in mtDNA mutator mice
Yuanjiu Lei, Camila Guerra Martinez, Sylvia Torres-Odio, Samantha L. Bell, Christine E. Birdwell, Joshua D. Bryant, Carl W. Tong, Robert O. Watson, Laura Ciaccia West, A. Phillip West
Science Advances.2021;[Epub] CrossRef - The potential role of Keap1-Nrf2 pathway in the pathogenesis of Alzheimer’s disease, type 2 diabetes, and type 2 diabetes-related Alzheimer’s disease
Ling He, Yi Sun
Metabolic Brain Disease.2021; 36(7): 1469. CrossRef - The impact of mitochondria on cancer treatment resistance
Michelle van der Merwe, Gustav van Niekerk, Carla Fourie, Manisha du Plessis, Anna-Mart Engelbrecht
Cellular Oncology.2021; 44(5): 983. CrossRef - Therapeutic Approaches Targeting Proteostasis in Kidney Disease and Fibrosis
Jia-Huang Chen, Chia-Hsien Wu, Chih-Kang Chiang
International Journal of Molecular Sciences.2021; 22(16): 8674. CrossRef - Coronary Large Conductance Ca2+-Activated K+ Channel Dysfunction in Diabetes Mellitus
Tong Lu, Hon-Chi Lee
Frontiers in Physiology.2021;[Epub] CrossRef - Ferroptosis: New Dawn for Overcoming the Cardio-Cerebrovascular Diseases
Meng-Yi Luo, Jian-Hui Su, Shao-Xin Gong, Na Liang, Wen-Qian Huang, Wei Chen, Ai-Ping Wang, Ying Tian
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Recombinant Human Growth Hormone Inhibits Lipotoxicity, Oxidative Stress, and Apoptosis in a Mouse Model of Diabetic Cardiomyopathy
Zuowei Pei, Xiang Wang, Chenguang Yang, Min Dong, Fang Wang, Juan Gambini
Oxidative Medicine and Cellular Longevity.2021; 2021: 1. CrossRef - Hydrogen sulfide mitigates myocardial inflammation by inhibiting nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation in diabetic rats
Qiang Jia, Shomaila Mehmood, Xiaofen Liu, Shanfeng Ma, Rui Yang
Experimental Biology and Medicine.2020; 245(3): 221. CrossRef - Heme oxygenase-1 in protozoan infections: A tale of resistance and disease tolerance
Rafael C. M. C. Silva, Leonardo H. Travassos, Claudia N. Paiva, Marcelo T. Bozza, Marc-Jan Gubbels
PLOS Pathogens.2020; 16(7): e1008599. CrossRef - Distinct Approaches of Raloxifene: Its Far-Reaching Beneficial Effects Implicating the HO-System
Denise Börzsei, Renáta Szabó, Alexandra Hoffmann, Médea Veszelka, Imre Pávó, Zsolt Turcsán, Csaba Viczián, Krisztina Kupai, Csaba Varga, Anikó Pósa
Biomolecules.2020; 10(3): 375. CrossRef - The Mitochondria: A Target of Polyphenols in the Treatment of Diabetic Cardiomyopathy
Humna Bhagani, Suzanne A. Nasser, Ali Dakroub, Ahmed F. El-Yazbi, Assaad A. Eid, Firas Kobeissy, Gianfranco Pintus, Ali H. Eid
International Journal of Molecular Sciences.2020; 21(14): 4962. CrossRef - The Effects of Dietary Supplements that Overactivate the Nrf2/ARE System
Robert E. Smith
Current Medicinal Chemistry.2020; 27(13): 2077. CrossRef - Allopurinol reduces oxidative stress and activates Nrf2/p62 to attenuate diabetic cardiomyopathy in rats
Jierong Luo, Dan Yan, Sisi Li, Shiming Liu, Fei Zeng, Chi Wai Cheung, Hong Liu, Michael G. Irwin, Huansen Huang, Zhengyuan Xia
Journal of Cellular and Molecular Medicine.2020; 24(2): 1760. CrossRef - Rg1 protects H9C2 cells from high glucose‐/palmitate‐induced injury via activation of AKT/GSK‐3β/Nrf2 pathway
Haitao Yu, Juan Zhen, Yang Yang, Jian Du, Jiyan Leng, Qian Tong
Journal of Cellular and Molecular Medicine.2020; 24(14): 8194. CrossRef - Ranolazine protects against diabetic cardiomyopathy by activating the NOTCH1/NRG1 pathway
Xi Chen, Long Ren, Xing Liu, Xi Sun, Chaorun Dong, Yanan Jiang, Ying Qin, Huan Qu, Jinfeng Jiao, Shuo Wang, Yunlong Bai, Baofeng Yang
Life Sciences.2020; 261: 118306. CrossRef - Low Concentration of Withaferin a Inhibits Oxidative Stress-Mediated Migration and Invasion in Oral Cancer Cells
Tzu-Jung Yu, Jen-Yang Tang, Fu Ou-Yang, Yen-Yun Wang, Shyng-Shiou F. Yuan, Kevin Tseng, Li-Ching Lin, Hsueh-Wei Chang
Biomolecules.2020; 10(5): 777. CrossRef - Protective Effect of Simplicillium sp. Ethyl Acetate Extract against High Glucose-Induced Oxidative Stress in HUVECs
Ting-Ting Tian, Qi-Rui Li, Shi-Quan Gan, Chu-Rui Chang, Xiang-Chun Shen
Evidence-Based Complementary and Alternative Medicine.2020; 2020: 1. CrossRef - Deneysel Diyabet Oluşturulan Sıçanlarda Kalp ve İskelet Kası Nrf2 Yapımı ve Oksidatif Stres Üzerine Melatoninin Etkisinin İncelenmesi
Salim ÖZENOĞLU, İnci TURAN, Hale SAYAN ÖZAÇMAK, Veysel Haktan ÖZAÇMAK
Turkish Journal of Diabetes and Obesity.2020; 4(1): 46. CrossRef - Nicorandil alleviates apoptosis in diabetic cardiomyopathy through PI3K/Akt pathway
Xuyang Wang, Jinyu Pan, Dian Liu, Mingjun Zhang, Xiaowei Li, Jingjing Tian, Ming Liu, Tao Jin, Fengshuang An
Journal of Cellular and Molecular Medicine.2019; 23(8): 5349. CrossRef - Bailcalin Protects against Diabetic Cardiomyopathy through Keap1/Nrf2/AMPK-Mediated Antioxidative and Lipid-Lowering Effects
Ran Li, Yuan Liu, Ying-guang Shan, Lu Gao, Fang Wang, Chun-guang Qiu
Oxidative Medicine and Cellular Longevity.2019; 2019: 1. CrossRef - Omega 3 rich diet modulates energy metabolism via GPR120-Nrf2 crosstalk in a novel antioxidant mouse model
Deborah Amos, Carla Cook, Nalini Santanam
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2019; 1864(4): 466. CrossRef - Increased placental expressions of nuclear factor erythroid 2–related factor 2 and antioxidant enzymes in gestational diabetes: Protective mechanisms against the placental oxidative stress?
Balachandiran Manoharan, Zachariah Bobby, Gowri Dorairajan, Sajini Elizabeth Jacob, Victorraj Gladwin, Vickneshwaran Vinayagam, Rajaa Muthu Packirisamy
European Journal of Obstetrics & Gynecology and Reproductive Biology.2019; 238: 78. CrossRef - Another look at phenolic compounds in cancer therapy the effect of polyphenols on ubiquitin-proteasome system
Aleksandra Golonko, Tomasz Pienkowski, Renata Swislocka, Ryszard Lazny, Marek Roszko, Wlodzimierz Lewandowski
European Journal of Medicinal Chemistry.2019; 167: 291. CrossRef - Hydrogen sulfide-mediated regulation of cell death signaling ameliorates adverse cardiac remodeling and diabetic cardiomyopathy
Sumit Kar, Tyler N. Kambis, Paras K. Mishra
American Journal of Physiology-Heart and Circulatory Physiology.2019; 316(6): H1237. CrossRef - Genomic and Genetic Approaches to Deciphering Acute Respiratory Distress Syndrome Risk and Mortality
Heather Lynn, Xiaoguang Sun, Nancy Casanova, Manuel Gonzales-Garay, Christian Bime, Joe G.N. Garcia
Antioxidants & Redox Signaling.2019; 31(14): 1027. CrossRef - Enhanced Keap1-Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling in mice
Gobinath Shanmugam, Anil Kumar Challa, Silvio H. Litovsky, Asokan Devarajan, Ding Wang, Dean P. Jones, Victor M. Darley-Usmar, Namakkal Soorappan Rajasekaran
Redox Biology.2019; 27: 101212. CrossRef - Effects of PP2A/Nrf2 on experimental diabetes mellitus-related cardiomyopathy by regulation of autophagy and apoptosis through ROS dependent pathway
Yanhui Guan, Lichun Zhou, Yu Zhang, Huiqin Tian, Anqi Li, Xiuzhen Han
Cellular Signalling.2019; 62: 109339. CrossRef - An Aza resveratrol–chalcone derivative 6b protects mice against diabetic cardiomyopathy by alleviating inflammation and oxidative stress
Shengban You, Jianchang Qian, Chuchu Sun, Hailing Zhang, Shiju Ye, Taiwei Chen, Zheng Xu, Jingying Wang, Weijian Huang, Guang Liang
Journal of Cellular and Molecular Medicine.2018; 22(3): 1931. CrossRef - Oxidative Stress in Mesenchymal Stem Cell Senescence: Regulation by Coding and Noncoding RNAs
Rosa Vono, Eva Jover Garcia, Gaia Spinetti, Paolo Madeddu
Antioxidants & Redox Signaling.2018; 29(9): 864. CrossRef - An overview of the emerging interface between cardiac metabolism, redox biology and the circadian clock
Rodrigo A. Peliciari-Garcia, Victor Darley-Usmar, Martin E. Young
Free Radical Biology and Medicine.2018; 119: 75. CrossRef - Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated inflammation both in vitro and in vivo
Yue Guo, Xiaodong Zhuang, Zena Huang, Jing Zou, Daya Yang, Xun Hu, Zhimin Du, Lichun Wang, Xinxue Liao
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2018; 1864(1): 238. CrossRef - Paradoxical cardiotoxicity of intraperitoneally-injected epigallocatechin gallate preparation in diabetic mice
Nora O. Abdel Rasheed, Lamiaa A. Ahmed, Dalaal M. Abdallah, Bahia M. El-Sayeh
Scientific Reports.2018;[Epub] CrossRef - Oxidative stress-induced miR-27a targets the redox gene nuclear factor erythroid 2-related factor 2 in diabetic embryopathy
Yang Zhao, Daoyin Dong, E. Albert Reece, Ashley R. Wang, Peixin Yang
American Journal of Obstetrics and Gynecology.2018; 218(1): 136.e1. CrossRef - Regulation on SIRT1-PGC-1α/Nrf2 pathway together with selective inhibition of aldose reductase makes compound hr5F a potential agent for the treatment of diabetic complications
Zhihua Wang, Sheng Yuan, Yanbing Li, Zhe Zhang, Wei Xiao, Dan Tang, Kaihe Ye, Zhijun Liu, Congcong Wang, Yixiong Zheng, Hong Nie, Heru Chen
Biochemical Pharmacology.2018; 150: 54. CrossRef - Fibroblast growth factor-21 prevents diabetic cardiomyopathy via AMPK-mediated antioxidation and lipid-lowering effects in the heart
Hong Yang, Anyun Feng, Sundong Lin, Lechu Yu, Xiufei Lin, Xiaoqing Yan, Xuemian Lu, Chi Zhang
Cell Death & Disease.2018;[Epub] CrossRef - Salusin-β contributes to oxidative stress and inflammation in diabetic cardiomyopathy
Ming-Xia Zhao, Bing Zhou, Li Ling, Xiao-Qing Xiong, Feng Zhang, Qi Chen, Yue-Hua Li, Yu-Ming Kang, Guo-Qing Zhu
Cell Death & Disease.2017; 8(3): e2690. CrossRef - Role of Nrf2 Signaling in the Regulation of Vascular BK Channel β1 Subunit Expression and BK Channel Function in High-Fat Diet–Induced Diabetic Mice
Tong Lu, Xiaojing Sun, Yong Li, Qiang Chai, Xiao-Li Wang, Hon-Chi Lee
Diabetes.2017; 66(10): 2681. CrossRef - Regulation of vascular large-conductance calcium-activated potassium channels by Nrf2 signalling
Yong Li, Xiao-Li Wang, Xiaojing Sun, Qiang Chai, Jingchao Li, Benjamin Thompson, Win-Kuang Shen, Tong Lu, Hon-Chi Lee
Diabetes and Vascular Disease Research.2017; 14(4): 353. CrossRef - Metallothionein Is Downstream of Nrf2 and Partially Mediates Sulforaphane Prevention of Diabetic Cardiomyopathy
Junlian Gu, Yanli Cheng, Hao Wu, Lili Kong, Shudong Wang, Zheng Xu, Zhiguo Zhang, Yi Tan, Bradley B. Keller, Honglan Zhou, Yuehui Wang, Zhonggao Xu, Lu Cai
Diabetes.2017; 66(2): 529. CrossRef - Kimchi methanol extracts attenuate hepatic steatosis induced by high cholesterol diet in low-density lipoprotein receptor knockout mice through inhibition of endoplasmic reticulum stress
Minji Woo, Mijeong Kim, Jeong Sook Noh, Yeong Ok Song
Journal of Functional Foods.2017; 32: 218. CrossRef - Aspalathin Protects the Heart against Hyperglycemia-Induced Oxidative Damage by Up-Regulating Nrf2 Expression
Phiwayinkosi Dludla, Christo Muller, Elizabeth Joubert, Johan Louw, M. Essop, Kwazi Gabuza, Samira Ghoor, Barbara Huisamen, Rabia Johnson
Molecules.2017; 22(1): 129. CrossRef - Intermittent hypoxia-induced cardiomyopathy and its prevention by Nrf2 and metallothionein
Shanshan Zhou, Xia Yin, Jingpeng Jin, Yi Tan, Daniel J. Conklin, Ying Xin, Zhiguo Zhang, Weixia Sun, Taixing Cui, Jun Cai, Yang Zheng, Lu Cai
Free Radical Biology and Medicine.2017; 112: 224. CrossRef - Inhibitory effects of rosmarinic acid on pterygium epithelial cells through redox imbalance and induction of extrinsic and intrinsic apoptosis
Ya-Yu Chen, Chia-Fang Tsai, Ming-Chu Tsai, Yu-Wen Hsu, Fung-Jou Lu
Experimental Eye Research.2017; 160: 96. CrossRef - Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus
Anne Lejay, Fei Fang, Rohan John, Julie A.D. Van, Meredith Barr, Fabien Thaveau, Nabil Chakfe, Bernard Geny, James W. Scholey
Journal of Molecular and Cellular Cardiology.2016; 91: 11. CrossRef - The Role of the Nrf2/ARE Antioxidant System in Preventing Cardiovascular Diseases
Robert Smith, Kevin Tran, Cynthia Smith, Miranda McDonald, Pushkar Shejwalkar, Kenji Hara
Diseases.2016; 4(4): 34. CrossRef - Prohibitin overexpression improves myocardial function in diabetic cardiomyopathy
Wen-qian Dong, Min Chao, Qing-hua Lu, Wei-li Chai, Wei Zhang, Xue-ying Chen, Er-shun Liang, Ling-bo Wang, Hong-liang Tian, Yu-guo Chen, Ming-xiang Zhang
Oncotarget.2016; 7(1): 66. CrossRef - Restoration of Nrf2 Signaling Normalizes the Regenerative Niche
Marc A. Soares, Oriana D. Cohen, Yee Cheng Low, Rita A. Sartor, Trevor Ellison, Utkarsh Anil, Lavinia Anzai, Jessica B. Chang, Pierre B. Saadeh, Piul S. Rabbani, Daniel J. Ceradini
Diabetes.2016; 65(3): 633. CrossRef - Myocardial transcription factors in diastolic dysfunction: clues for model systems and disease
Alexander T. Mikhailov, Mario Torrado
Heart Failure Reviews.2016; 21(6): 783. CrossRef - Antioxidant Properties of Whole Body Periodic Acceleration (pGz)
Arkady Uryash, Jorge Bassuk, Paul Kurlansky, Francisco Altamirano, Jose R. Lopez, Jose A. Adams, Guillermo López Lluch
PLOS ONE.2015; 10(7): e0131392. CrossRef - Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice
Ji-Young Ann, Hyeyoon Eo, Yunsook Lim
Genes & Nutrition.2015;[Epub] CrossRef - Oxidative Stress and Human Hypertension: Vascular Mechanisms, Biomarkers, and Novel Therapies
Augusto C. Montezano, Maria Dulak-Lis, Sofia Tsiropoulou, Adam Harvey, Ana M. Briones, Rhian M. Touyz
Canadian Journal of Cardiology.2015; 31(5): 631. CrossRef - Haeme oxygenase signalling pathway: implications for cardiovascular disease
Laura E. Fredenburgh, Allison A. Merz, Susan Cheng
European Heart Journal.2015; 36(24): 1512. CrossRef
- Natural Products and Health
- FGF21 as a Stress Hormone: The Roles of FGF21 in Stress Adaptation and the Treatment of Metabolic Diseases
- Kook Hwan Kim, Myung-Shik Lee
- Diabetes Metab J. 2014;38(4):245-251. Published online August 20, 2014
- DOI: https://doi.org/10.4093/dmj.2014.38.4.245
- 5,354 View
- 79 Download
- 103 Web of Science
- 102 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Fibroblast growth factor 21 (FGF21) is an endocrine hormone that is primarily expressed in the liver and exerts beneficial effects on obesity and related metabolic diseases. In addition to its remarkable pharmacologic actions, the physiological roles of FGF21 include the maintenance of energy homeostasis in the body in conditions of metabolic or environmental stress. The expression of FGF21 is induced in multiple organs in response to diverse physiological or pathological stressors, such as starvation, nutrient excess, autophagy deficiency, mitochondrial stress, exercise, and cold exposure. Thus, the FGF21 induction caused by stress plays an important role in adaptive response to these stimuli. Here, we highlight our current understanding of the functional importance of the induction of FGF21 by diverse stressors as a feedback mechanism that prevents excessive stress.
-
Citations
Citations to this article as recorded by- Fibroblast growth factor 21: An emerging pleiotropic regulator of lipid metabolism and the metabolic network
Shuo Li, Tiande Zou, Jun Chen, Jiaming Li, Jinming You
Genes & Diseases.2024; 11(3): 101064. CrossRef - From Beats to Metabolism: the Heart at the Core of Interorgan Metabolic Cross Talk
Rafael Romero-Becera, Ayelén M. Santamans, Alba C. Arcones, Guadalupe Sabio
Physiology.2024; 39(2): 98. CrossRef - Beneficial Effects of Low-Grade Mitochondrial Stress on Metabolic Diseases and Aging
Se Hee Min, Gil Myoung Kang, Jae Woo Park, Min-Seon Kim
Yonsei Medical Journal.2024; 65(2): 55. CrossRef - GDF15 is a dynamic biomarker of the integrated stress response in the central nervous system
Jyoti Asundi, Chunlian Zhang, Diana Donnelly‐Roberts, Josè Zavala Solorio, Malleswari Challagundla, Caitlin Connelly, Christina Boch, Jun Chen, Mario Richter, Mohammad Mehdi Maneshi, Andrew M. Swensen, Lauren Lebon, Raphael Schiffmann, Subhabrata Sanyal,
CNS Neuroscience & Therapeutics.2024;[Epub] CrossRef - Deciphering adipose development: Function, differentiation and regulation
Ge Guo, Wanli Wang, Mengjie Tu, Binbin Zhao, Jiayang Han, Jiali Li, Yanbing Pan, Jie Zhou, Wen Ma, Yi Liu, Tiantian Sun, Xu Han, Yang An
Developmental Dynamics.2024;[Epub] CrossRef - Inflammatory liver diseases and susceptibility to sepsis
Hong Lu
Clinical Science.2024; 138(7): 435. CrossRef - Fibroblast growth factor 21 and bone homeostasis
Yan Tang, Mei Zhang
Biomedical Journal.2023; 46(4): 100548. CrossRef - Chronic docosahexaenoic acid supplementation improves metabolic plasticity in subcutaneous adipose tissue of aged obese female mice
Elisa Félix-Soriano, Neira Sáinz, Marta Fernández-Galilea, Eva Gil-Iturbe, Jon Celay, José A. Martínez-Climent, María J. Moreno-Aliaga
The Journal of Nutritional Biochemistry.2023; 111: 109153. CrossRef - Fibroblast Growth Factor–Based Pharmacotherapies for the Treatment of Obesity-Related Metabolic Complications
Leigang Jin, Ranyao Yang, Leiluo Geng, Aimin Xu
Annual Review of Pharmacology and Toxicology.2023; 63(1): 359. CrossRef - The effect of FGF21 gene polymorphism (g. 940C/T) on biochemical metabolic parameters in blood serum of holstein cattle
N. Yu. Safina, Sh. K. Shakrov, E. R. Gaynutdinova, Z. F. Fattakhova
International Journal of Veterinary Medicine.2023; (4): 314. CrossRef - Fibroblast growth factor 21 is expressed and secreted from skeletal muscle following electrical stimulation via extracellular ATP activation of the PI3K/Akt/mTOR signaling pathway
Manuel Arias-Calderón, Mariana Casas, Julián Balanta-Melo, Camilo Morales-Jiménez, Nadia Hernández, Paola Llanos, Enrique Jaimovich, Sonja Buvinic
Frontiers in Endocrinology.2023;[Epub] CrossRef - Fibroblast growth factor 21 as a potential master regulator in metabolic disorders
Aayushi Velingkar, Sugunakar Vuree, Pranav Kumar Prabhakar, Rajender Rao Kalashikam, Aparna Banerjee, Suresh Kondeti
American Journal of Physiology-Endocrinology and Metabolism.2023; 324(5): E409. CrossRef - Association of NAFLD with FGF21 Polygenic Hazard Score, and Its Interaction with Protein Intake Level in Korean Adults
Hae Jin Lee, Jinyoung Shon, Yoon Jung Park
Nutrients.2023; 15(10): 2385. CrossRef - Blood and liver telomere length, mitochondrial DNA copy number, and hepatic gene expression of mitochondrial dynamics in mid-lactation cows supplemented with l-carnitine under systemic inflammation
S. Häussler, M.H. Ghaffari, K. Seibt, H. Sadri, M. Alaedin, K. Huber, J. Frahm, S. Dänicke, H. Sauerwein
Journal of Dairy Science.2023; 106(12): 9822. CrossRef - Pharmacological Effects of Fibroblast Growth Factor 21 (FGF21) оn Carbohydrate-Lipid Metabolism: Sex Dependence
N. M. Bazhan, E. N. Makarova
Успехи физиологических наук.2023; 54(4): 93. CrossRef - The Nuanced Metabolic Functions of Endogenous FGF21 Depend on the Nature of the Stimulus, Tissue Source, and Experimental Model
Redin A. Spann, Christopher D. Morrison, Laura J. den Hartigh
Frontiers in Endocrinology.2022;[Epub] CrossRef - Long-Term Dietary Taurine Lowers Plasma Levels of Cholesterol and Bile Acids
Ryoma Tagawa, Masaki Kobayashi, Misako Sakurai, Maho Yoshida, Hiroki Kaneko, Yuhei Mizunoe, Yuka Nozaki, Naoyuki Okita, Yuka Sudo, Yoshikazu Higami
International Journal of Molecular Sciences.2022; 23(3): 1793. CrossRef - Endocrine Fibroblast Growth Factors in Relation to Stress Signaling
Makoto Shimizu, Ryuichiro Sato
Cells.2022; 11(3): 505. CrossRef - Histone Deacetylases as Modulators of the Crosstalk Between Skeletal Muscle and Other Organs
Alessandra Renzini, Marco D’Onghia, Dario Coletti, Viviana Moresi
Frontiers in Physiology.2022;[Epub] CrossRef - Multidimensional Biomarker Analysis Including Mitochondrial Stress Indicators for Nonalcoholic Fatty Liver Disease
Eunha Chang, Jae Seung Chang, In Deok Kong, Soon Koo Baik, Moon Young Kim, Kyu-Sang Park
Gut and Liver.2022; 16(2): 171. CrossRef - FGF21: A Novel Regulator of Glucose and Lipid Metabolism and
Whole-Body Energy Balance
Ewa Szczepańska, Małgorzata Gietka-Czernel
Hormone and Metabolic Research.2022; 54(04): 203. CrossRef - Exercise, Mitohormesis, and Mitochondrial ORF of the 12S rRNA Type-C (MOTS-c)
Tae Kwan Yoon, Chan Hee Lee, Obin Kwon, Min-Seon Kim
Diabetes & Metabolism Journal.2022; 46(3): 402. CrossRef - Counteracting health risks by Modulating Homeostatic Signaling
Junqiang J. Tian, Mark Levy, Xuekai Zhang, Robert Sinnott, Rolando Maddela
Pharmacological Research.2022; 182: 106281. CrossRef - Hesperidin abrogates bisphenol A endocrine disruption through binding with fibroblast growth factor 21 (FGF-21), α-amylase and α-glucosidase: an in silico molecular study
P.M. Aja, J.N. Awoke, P.C. Agu, A.E. Adegboyega, E.M. Ezeh, I.O. Igwenyi, O.U. Orji, O.G. Ani, B.A. Ale, U.A. Ibiam
Journal of Genetic Engineering and Biotechnology.2022; 20(1): 84. CrossRef - Circulating FGF21 vs. Stress Markers in Girls during Childhood and Adolescence, and in Their Caregivers: Intriguing Inter-Relations between Overweight/Obesity, Emotions, Behavior, and the Cared-Caregiver Relationship
Eirini V. Christaki, Panagiota Pervanidou, Ioannis Papassotiriou, Aimilia Mantzou, Giorgos Giannakakis, Dario Boschiero, George P. Chrousos
Children.2022; 9(6): 821. CrossRef - α‐Lipoic acid up‐regulates gene expression but reduces protein levels of fibroblast growth factor 21 in HepG2 cells
Xiaochun Zhang, Yanyan Zhao, Xiangyan Liang, Lijun Zhang, Ke Li, Zhuo Sun, Yu‐Feng Zhao
Basic & Clinical Pharmacology & Toxicology.2022; 131(4): 270. CrossRef - Multi-organ FGF21-FGFR1 signaling in metabolic health and disease
Namrita Kaur, Sanskruti Ravindra Gare, Jiahan Shen, Rida Raja, Oveena Fonseka, Wei Liu
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Management of patients with neuropathic pain
Eun Joo Choi
Journal of the Korean Medical Association.2022; 65(8): 505. CrossRef - FGF21–Sirtuin 3 Axis Confers the Protective Effects of Exercise Against Diabetic Cardiomyopathy by Governing Mitochondrial Integrity
Leigang Jin, Leiluo Geng, Lei Ying, Lingling Shu, Kevin Ye, Ranyao Yang, Yan Liu, Yao Wang, Yin Cai, Xue Jiang, Qin Wang, Xingqun Yan, Boya Liao, Jie Liu, Fuyu Duan, Gary Sweeney, Connie Wai Hong Woo, Yu Wang, Zhengyuan Xia, Qizhou Lian, Aimin Xu
Circulation.2022; 146(20): 1537. CrossRef - Performance and Metabolic, Inflammatory, and Oxidative Stress-Related Parameters in Early Lactating Dairy Cows with High and Low Hepatic FGF21 Expression
Denise K. Gessner, Lena M. Sandrock, Erika Most, Christian Koch, Robert Ringseis, Klaus Eder
Animals.2022; 13(1): 131. CrossRef - Relationship between FGF21 and drug or nondrug therapy of type 2 diabetes mellitus
Chang Guo, Li Zhao, Yanyan Li, Xia Deng, Guoyue Yuan
Journal of Cellular Physiology.2021; 236(1): 55. CrossRef - Skeletal Muscle and Bone – Emerging Targets of Fibroblast Growth Factor-21
Hui Sun, Matthew Sherrier, Hongshuai Li
Frontiers in Physiology.2021;[Epub] CrossRef - Transthyretin contributes to insulin resistance and diminishes exercise-induced insulin sensitivity in obese mice by inhibiting AMPK activity in skeletal muscle
Yingzi He, Ruojun Qiu, Beibei Wu, Weiwei Gui, Xihua Lin, Hong Li, Fenping Zheng
American Journal of Physiology-Endocrinology and Metabolism.2021; 320(4): E808. CrossRef - Metabolic Messengers: FGF21
Kyle H. Flippo, Matthew J. Potthoff
Nature Metabolism.2021; 3(3): 309. CrossRef - Circulating Fibroblast Growth Factor-21 Levels in Rheumatoid Arthritis: Associations With Disease Characteristics, Body Composition, and Physical Functioning
Patrick W. Gould, Babette S. Zemel, Elena G. Taratuta, Joshua F. Baker
The Journal of Rheumatology.2021; 48(4): 504. CrossRef - The transcription factors CREBH, PPARa, and FOXO1 as critical hepatic mediators of diet-induced metabolic dysregulation
Zhao Yang, Katherine Roth, Manisha Agarwal, Wanqing Liu, Michael C. Petriello
The Journal of Nutritional Biochemistry.2021; 95: 108633. CrossRef - Low‐Level Radiofrequency Exposure Induces Vasoconstriction in Rats
Thi Cuc Mai, Anne Braun, Veronique Bach, Amandine Pelletier, Rene de Seze
Bioelectromagnetics.2021; 42(6): 455. CrossRef - Anterograde regulation of mitochondrial genes and FGF21 signaling by hepatic LSD1
Yang Cao, Lingyi Tang, Kang Du, Kitt Paraiso, Qiushi Sun, Zhengxia Liu, Xiaolong Ye, Yuan Fang, Fang Yuan, Hank Chen, Yumay Chen, Xiaorong Wang, Clinton Yu, Ira L. Blitz, Ping H. Wang, Lan Huang, Haibo Cheng, Xiang Lu, Ken W.Y. Cho, Marcus Seldin, Zhuyuan
JCI Insight.2021;[Epub] CrossRef - Fibroblast growth factor 21 attenuates iron overload-induced liver injury and fibrosis by inhibiting ferroptosis
Aimin Wu, Bin Feng, Jie Yu, Lijun Yan, Lianqiang Che, Yong Zhuo, Yuheng Luo, Bing Yu, De Wu, Daiwen Chen
Redox Biology.2021; 46: 102131. CrossRef - Stress-induced FGF21 and GDF15 in obesity and obesity resistance
Susanne Keipert, Mario Ost
Trends in Endocrinology & Metabolism.2021; 32(11): 904. CrossRef - Fibroblast growth factor 21 in dairy cows: current knowledge and potential relevance
Klaus Eder, Denise K. Gessner, Robert Ringseis
Journal of Animal Science and Biotechnology.2021;[Epub] CrossRef - Weight regain after bariatric surgery: Promoters and potential predictors
Hala Mourad Demerdash
World Journal of Meta-Analysis.2021; 9(5): 438. CrossRef - αKlotho attenuates cardiac hypertrophy and increases myocardial fibroblast growth factor 21 expression in uremic rats
Paulo Giovani de Albuquerque Suassuna, Paula Marocolo Cherem, Bárbara Bruna de Castro, Edgar Maquigussa, Marco Antonio Cenedeze, Júlio Cesar Moraes Lovisi, Melani Ribeiro Custódio, Helady Sanders-Pinheiro, Rogério Baumgratz de Paula
Experimental Biology and Medicine.2020; 245(1): 66. CrossRef - Effect of Fibroblast Growth Factor 21 on the Development of Atheromatous Plaque and Lipid Metabolic Profiles in an Atherosclerosis-Prone Mouse Model
Hyo Jin Maeng, Gha Young Lee, Jae Hyun Bae, Soo Lim
International Journal of Molecular Sciences.2020; 21(18): 6836. CrossRef - A Land of Controversy: Fibroblast Growth Factor-23 and Uremic Cardiac Hypertrophy
Jing-Fu Bao, Pan-Pan Hu, Qin-Ying She, Aiqing Li
Journal of the American Society of Nephrology.2020; 31(7): 1423. CrossRef - Possible associations between plasma fibroblast growth factor 21 levels and cognition in bipolar disorder
Favour Omileke, Sayuri Ishiwata, Junko Matsuo, Fuyuko Yoshida, Shinsuke Hidese, Kotaro Hattori, Hiroshi Kunugi
Neuropsychopharmacology Reports.2020; 40(2): 175. CrossRef - FGF21-protection against fructose-induced lipid accretion and oxidative stress is influenced by maternal nutrition in male progeny
Elena Fauste, Silvia Rodrigo, Lourdes Rodríguez, Cristina Donis, Antonia García, Coral Barbas, Juan J. Álvarez-Millán, María I. Panadero, Paola Otero, Carlos Bocos
Journal of Functional Foods.2020; 64: 103676. CrossRef - Fibroblast growth factor 21 and grow differentiation factor 15 are sensitive biomarkers of mitochondrial diseases due to mitochondrial transfer-RNA mutations and mitochondrial DNA deletions
Patrizia Formichi, Nastasia Cardone, Ilaria Taglia, Elena Cardaioli, Simona Salvatore, Annalisa Lo Gerfo, Costanza Simoncini, Vincenzo Montano, Gabriele Siciliano, Michelangelo Mancuso, Alessandro Malandrini, Antonio Federico, Maria Teresa Dotti
Neurological Sciences.2020; 41(12): 3653. CrossRef - Novel Medicinal Mushroom Blend as a Promising Supplement in Integrative Oncology: A Multi-Tiered Study using 4T1 Triple-Negative Mouse Breast Cancer Model
Elisa Roda, Fabrizio De Luca, Carmine Di Iorio, Daniela Ratto, Stella Siciliani, Beatrice Ferrari, Filippo Cobelli, Giuseppina Borsci, Erica Cecilia Priori, Silvia Chinosi, Andrea Ronchi, Renato Franco, Raffaele Di Francia, Massimiliano Berretta, Carlo Al
International Journal of Molecular Sciences.2020; 21(10): 3479. CrossRef - High‐protein diet more effectively reduces hepatic fat than low‐protein diet despite lower autophagy and FGF21 levels
Chenchen Xu, Mariya Markova, Nicole Seebeck, Anne Loft, Silke Hornemann, Thomas Gantert, Stefan Kabisch, Kathleen Herz, Jennifer Loske, Mario Ost, Verena Coleman, Frederick Klauschen, Anke Rosenthal, Volker Lange, Jürgen Machann, Susanne Klaus, Tilman Gru
Liver International.2020; 40(12): 2982. CrossRef - Recharacterizing the Metabolic State of Energy Balance in Thrifty and Spendthrift Phenotypes
Tim Hollstein, Alessio Basolo, Takafumi Ando, Susanne B Votruba, Mary Walter, Jonathan Krakoff, Paolo Piaggi
The Journal of Clinical Endocrinology & Metabolism.2020; 105(5): 1375. CrossRef - Targeting of Secretory Proteins as a Therapeutic Strategy for Treatment of Nonalcoholic Steatohepatitis (NASH)
Kyeongjin Kim, Kook Hwan Kim
International Journal of Molecular Sciences.2020; 21(7): 2296. CrossRef - Discovery of a novel fibroblast activation protein (FAP) inhibitor, BR103354, with anti-diabetic and anti-steatotic effects
Jae Min Cho, Eun Hee Yang, Wenying Quan, Eun Hye Nam, Hyae Gyeong Cheon
Scientific Reports.2020;[Epub] CrossRef - Spontaneous ketonuria and risk of incident diabetes: a 12 year prospective study
Gyuri Kim, Sang-Guk Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Ele Ferrannini, Yong-ho Lee, Nam H. Cho
Diabetologia.2019; 62(5): 779. CrossRef - Combined docosahexaenoic acid and thyroid hormone supplementation as a protocol supporting energy supply to precondition and afford protection against metabolic stress situations
Luis A. Videla
IUBMB Life.2019; 71(9): 1211. CrossRef - Effects of exercise on brown and beige adipocytes
Revati S. Dewal, Kristin I. Stanford
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2019; 1864(1): 71. CrossRef - Manipulating mtDNA in vivo reprograms metabolism via novel response mechanisms
Diana Bahhir, Cagri Yalgin, Liina Ots, Sampsa Järvinen, Jack George, Alba Naudí, Tatjana Krama, Indrikis Krams, Mairi Tamm, Ana Andjelković, Eric Dufour, Jose M. González de Cózar, Mike Gerards, Mikael Parhiala, Reinald Pamplona, Howard T. Jacobs, Priit J
PLOS Genetics.2019; 15(10): e1008410. CrossRef - The Level of FGF 21 as a New Risk Factor for the Occurrence of Cardiometabolic Disorders amongst the Psoriatic Patients
Paulina Kiluk, Anna Baran, Tomasz W. Kaminski, Magdalena Maciaszek, Iwona Flisiak
Journal of Clinical Medicine.2019; 8(12): 2206. CrossRef - Docosahexaenoic acid‐thyroid hormone combined protocol as a novel approach to metabolic stress disorders: Relation to mitochondrial adaptation via liver PGC‐1α and sirtuin1 activation
Romina Vargas, Bárbara Riquelme, Javier Fernández, Daniela Álvarez, Ignacio F. Pérez, Pamela Cornejo, Virginia Fernández, Luis A. Videla
BioFactors.2019; 45(2): 271. CrossRef - Fibroblast growth factor 21 predicts outcome in community-acquired pneumonia: secondary analysis of two randomised controlled trials
Fahim Ebrahimi, Carole Wolffenbuttel, Claudine A. Blum, Christine Baumgartner, Beat Mueller, Philipp Schuetz, Christian Meier, Marius Kraenzlin, Mirjam Christ-Crain, Matthias Johannes Betz
European Respiratory Journal.2019; 53(2): 1800973. CrossRef - Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat
Huating Li, Guangyu Wu, Qichen Fang, Mingliang Zhang, Xiaoyan Hui, Bin Sheng, Liang Wu, Yuqian Bao, Peng Li, Aimin Xu, Weiping Jia
Nature Communications.2018;[Epub] CrossRef - Effects of supplementing rumen-protected niacin on fiber composition and metabolism of skeletal muscle in dairy cows during early lactation
J.O. Zeitz, A. Weber, E. Most, W. Windisch, C. Bolduan, J. Geyer, F.-J. Romberg, C. Koch, K. Eder
Journal of Dairy Science.2018; 101(9): 8004. CrossRef - The mitochondrial unfolded protein response and mitohormesis: a perspective on metabolic diseases
Hyon-Seung Yi, Joon Young Chang, Minho Shong
Journal of Molecular Endocrinology.2018; 61(3): R91. CrossRef - Berberine-induced activation of AMPK increases hepatic FGF21 expression via NUR77
Feiye Zhou, Mengyao Bai, Yuqing Zhang, Qin Zhu, Linlin Zhang, Qi Zhang, Shushu Wang, Kecheng Zhu, Yun Liu, Xiao Wang, Libin Zhou
Biochemical and Biophysical Research Communications.2018; 495(2): 1936. CrossRef - FGF21 Attenuates High-Fat Diet-Induced Cognitive Impairment via Metabolic Regulation and Anti-inflammation of Obese Mice
Qingzhi Wang, Jing Yuan, Zhanyang Yu, Li Lin, Yinghua Jiang, Zeyuan Cao, Pengwei Zhuang, Michael J. Whalen, Bo Song, Xiao-Jie Wang, Xiaokun Li, Eng H. Lo, Yuming Xu, Xiaoying Wang
Molecular Neurobiology.2018; 55(6): 4702. CrossRef - Leptin Mediates a Glucose-Fatty Acid Cycle to Maintain Glucose Homeostasis in Starvation
Rachel J. Perry, Yongliang Wang, Gary W. Cline, Aviva Rabin-Court, Joongyu D. Song, Sylvie Dufour, Xian Man Zhang, Kitt Falk Petersen, Gerald I. Shulman
Cell.2018; 172(1-2): 234. CrossRef - Improvement of Lipid and Glucose Metabolism by Capsiate in Palmitic Acid-Treated HepG2 Cells via Activation of the AMPK/SIRT1 Signaling Pathway
Yufan Zang, Li Fan, Jihua Chen, Ruixue Huang, Hong Qin
Journal of Agricultural and Food Chemistry.2018; 66(26): 6772. CrossRef - Fibroblast growth factor 21 as a biomarker for long‐term complications in organic acidemias
F. Molema, E. H. Jacobs, W. Onkenhout, G. C. Schoonderwoerd, J. G. Langendonk, Monique Williams
Journal of Inherited Metabolic Disease.2018; 41(6): 1179. CrossRef - The mitochondrial UPR: mechanisms, physiological functions and implications in ageing
Tomer Shpilka, Cole M. Haynes
Nature Reviews Molecular Cell Biology.2018; 19(2): 109. CrossRef - FGF21 Is Associated with Metabolic Effects and Treatment Response in Depressed Bipolar II Disorder Patients Treated with Valproate
Hui Hua Chang, Po See Chen, Yung Wen Cheng, Tzu-Yun Wang, Yen Kuang Yang, Ru-Band Lu
International Journal of Neuropsychopharmacology.2018; 21(4): 319. CrossRef - Circulating Fibroblast Growth Factor 21 is Associated with Subsequent Renal Injury Events in Patients Undergoing Coronary Angiography
Cheng-Hsueh Wu, Ruey-Hsing Chou, Chin-Sung Kuo, Po-Hsun Huang, Chun-Chin Chang, Hsin-Bang Leu, Chin-Chou Huang, Jaw-Wen Chen, Shing-Jong Lin
Scientific Reports.2018;[Epub] CrossRef - Fibroblast Growth Factor 21: A Versatile Regulator of Metabolic Homeostasis
Lucas D. BonDurant, Matthew J. Potthoff
Annual Review of Nutrition.2018; 38(1): 173. CrossRef - Endocrine Regulator rFGF21 (Recombinant Human Fibroblast Growth Factor 21) Improves Neurological Outcomes Following Focal Ischemic Stroke of Type 2 Diabetes Mellitus Male Mice
Yinghua Jiang, Ning Liu, Qingzhi Wang, Zhanyang Yu, Li Lin, Jing Yuan, Shuzhen Guo, Bum Ju Ahn, Xiao-Jie Wang, Xiaokun Li, Eng H. Lo, Xiaochuan Sun, Xiaoying Wang
Stroke.2018; 49(12): 3039. CrossRef - Restricting branched‐chain amino acids: an approach to improve metabolic health
Jacob G. Anderson, Kenzie Hintze, Erik D. Marchant
The Journal of Physiology.2018; 596(13): 2469. CrossRef - Dietary Manipulations That Induce Ketosis Activate the HPA Axis in Male Rats and Mice: A Potential Role for Fibroblast Growth Factor-21
Karen K Ryan, Amy E B Packard, Karlton R Larson, Jayna Stout, Sarah M Fourman, Abigail M K Thompson, Kristen Ludwick, Kirk M Habegger, Kerstin Stemmer, Nobuyuki Itoh, Diego Perez-Tilve, Matthias H Tschöp, Randy J Seeley, Yvonne M Ulrich-Lai
Endocrinology.2018; 159(1): 400. CrossRef - Mitochondrial dysfunction in cancer: Potential roles of ATF5 and the mitochondrial UPR
Pan Deng, Cole M. Haynes
Seminars in Cancer Biology.2017; 47: 43. CrossRef - Modulation of energy balance by fibroblast growth factor 21
Daniel Cuevas-Ramos, Carlos A. Aguilar-Salinas
Hormone Molecular Biology and Clinical Investigation.2017;[Epub] CrossRef - Fibroblast Growth Factor 21 Mimetics for Treating Atherosclerosis
Kelvin H. M. Kwok, Karen S. L. Lam
Endocrinology and Metabolism.2017; 32(2): 145. CrossRef - The U-shaped relationship between fibroblast growth factor 21 and microvascular complication in type 2 diabetes mellitus
Chan-Hee Jung, Sang-Hee Jung, Bo-Yeon Kim, Chul-Hee Kim, Sung-Koo Kang, Ji-Oh Mok
Journal of Diabetes and its Complications.2017; 31(1): 134. CrossRef - A combined docosahexaenoic acid–thyroid hormone protocol upregulates rat liver β-Klotho expression and downstream components of FGF21 signaling as a potential novel approach to metabolic stress conditions
R. Vargas, B. Riquelme, J. Fernández, L. A. Videla
Food & Function.2017; 8(11): 3980. CrossRef - Anti-inflammatory effects of exercise training in adipose tissue do not require FGF21
Jay W Porter, Joe L Rowles, Justin A Fletcher, Terese M Zidon, Nathan C Winn, Leighton T McCabe, Young-Min Park, James W Perfield, John P Thyfault, R Scott Rector, Jaume Padilla, Victoria J Vieira-Potter
Journal of Endocrinology.2017; 235(2): 97. CrossRef - Hepatic FXR/SHP axis modulates systemic glucose and fatty acid homeostasis in aged mice
Kang Ho Kim, Sungwoo Choi, Ying Zhou, Eun Young Kim, Jae Man Lee, Pradip K. Saha, Sayeepriyadarshini Anakk, David D. Moore
Hepatology.2017; 66(2): 498. CrossRef - Association between circulating fibroblast growth factor 21 and mortality in end-stage renal disease
Marina Kohara, Takahiro Masuda, Kazuhiro Shiizaki, Tetsu Akimoto, Yuko Watanabe, Sumiko Honma, Chuji Sekiguchi, Yasuharu Miyazawa, Eiji Kusano, Yoshinobu Kanda, Yasushi Asano, Makoto Kuro-o, Daisuke Nagata, Tatsuo Shimosawa
PLOS ONE.2017; 12(6): e0178971. CrossRef - Analysis of hepatic transcript profile and plasma lipid profile in early lactating dairy cows fed grape seed and grape marc meal extract
Denise K. Gessner, Anne Winkler, Christian Koch, Georg Dusel, Gerhard Liebisch, Robert Ringseis, Klaus Eder
BMC Genomics.2017;[Epub] CrossRef - The FGF21-CCL11 Axis Mediates Beiging of White Adipose Tissues by Coupling Sympathetic Nervous System to Type 2 Immunity
Zhe Huang, Ling Zhong, Jimmy Tsz Hang Lee, Jialiang Zhang, Donghai Wu, Leiluo Geng, Yu Wang, Chi-Ming Wong, Aimin Xu
Cell Metabolism.2017; 26(3): 493. CrossRef - Fibroblast growth factor 21 and its novel association with oxidative stress
Miguel Ángel Gómez-Sámano, Mariana Grajales-Gómez, Julia María Zuarth-Vázquez, Ma. Fernanda Navarro-Flores, Mayela Martínez-Saavedra, Óscar Alfredo Juárez-León, Mariana G. Morales-García, Víctor Manuel Enríquez-Estrada, Francisco J. Gómez-Pérez, Daniel Cu
Redox Biology.2017; 11: 335. CrossRef - FGF21 activates AMPK signaling: impact on metabolic regulation and the aging process
Antero Salminen, Anu Kauppinen, Kai Kaarniranta
Journal of Molecular Medicine.2017; 95(2): 123. CrossRef - The Role of Autophagy in Critical Illness-induced Liver Damage
Steven E. Thiessen, Inge Derese, Sarah Derde, Thomas Dufour, Lies Pauwels, Youri Bekhuis, Isabel Pintelon, Wim Martinet, Greet Van den Berghe, Ilse Vanhorebeek
Scientific Reports.2017;[Epub] CrossRef - Implication of hepatokines in metabolic disorders and cardiovascular diseases
Tae Woo Jung, Hye Jin Yoo, Kyung Mook Choi
BBA Clinical.2016; 5: 108. CrossRef - Upregulation of rat liver PPARα‐FGF21 signaling by a docosahexaenoic acid and thyroid hormone combined protocol
Luis A. Videla, Virginia Fernández, Romina Vargas, Pamela Cornejo, Gladys Tapia, Nelson Varela, Rodrigo Valenzuela, Allan Arenas, Javier Fernández, María C. Hernández‐Rodas, Bárbara Riquelme
BioFactors.2016; 42(6): 638. CrossRef - Fibroblast Growth Factor 21 Protects against Atherosclerosis via Fine-Tuning the Multiorgan Crosstalk
Leigang Jin, Zhuofeng Lin, Aimin Xu
Diabetes & Metabolism Journal.2016; 40(1): 22. CrossRef - Hepatic Fgf21 Expression Is Repressed after Simvastatin Treatment in Mice
Panos Ziros, Zoi Zagoriti, George Lagoumintzis, Venetsana Kyriazopoulou, Ralitsa P. Iskrenova, Evagelia I. Habeos, Gerasimos P. Sykiotis, Dionysios V. Chartoumpekis, Ioannis G Habeos, Kostas Pantopoulos
PLOS ONE.2016; 11(9): e0162024. CrossRef - Metabolic fibroblast growth factors (FGFs): Mediators of energy homeostasis
Kathleen R. Markan, Matthew J. Potthoff
Seminars in Cell & Developmental Biology.2016; 53: 85. CrossRef - miR‐212 downregulation contributes to the protective effect of exercise against non‐alcoholic fatty liver via targeting FGF‐21
Junjie Xiao, Yihua Bei, Jingqi Liu, Jasmina Dimitrova‐Shumkovska, Dapeng Kuang, Qiulian Zhou, Jin Li, Yanning Yang, Yang Xiang, Fei Wang, Changqing Yang, Wenzhuo Yang
Journal of Cellular and Molecular Medicine.2016; 20(2): 204. CrossRef - Fibroblast growth factor 21 and exercise-induced hepatic mitochondrial adaptations
Justin A. Fletcher, Melissa A. Linden, Ryan D. Sheldon, Grace M. Meers, E. Matthew Morris, Anthony Butterfield, James W. Perfield, John P. Thyfault, R. Scott Rector
American Journal of Physiology-Gastrointestinal and Liver Physiology.2016; 310(10): G832. CrossRef - New adipokines
Bruno Fève, Claire Bastard, Soraya Fellahi, Jean-Philippe Bastard, Jacqueline Capeau
Annales d'Endocrinologie.2016; 77(1): 49. CrossRef - Stress Signaling Between Organs in Metazoa
Edward Owusu-Ansah, Norbert Perrimon
Annual Review of Cell and Developmental Biology.2015; 31(1): 497. CrossRef - Dietary restriction in obese children and its relation with eating behavior, fibroblast growth factor 21 and leptin: a prospective clinical intervention study
Lorena del Rocío Ibarra-Reynoso, Liudmila Pisarchyk, Elva Leticia Pérez-Luque, Ma. Eugenia Garay-Sevilla, Juan Manuel Malacara
Nutrition & Metabolism.2015;[Epub] CrossRef - FGF21 as a mediator of adaptive responses to stress and metabolic benefits of anti-diabetic drugs
Kook Hwan Kim, Myung-Shik Lee
Journal of Endocrinology.2015; 226(1): R1. CrossRef - Effects of a plant product consisting of green tea and curcuma extract on milk production and the expression of hepatic genes involved in endoplasmic stress response and inflammation in dairy cows
Anne Winkler, Denise K. Gessner, Christian Koch, Franz-Josef Romberg, Georg Dusel, Eva Herzog, Erika Most, Klaus Eder
Archives of Animal Nutrition.2015; 69(6): 425. CrossRef - The effect of grape seed and grape marc meal extract on milk performance and the expression of genes of endoplasmic reticulum stress and inflammation in the liver of dairy cows in early lactation
D.K. Gessner, C. Koch, F.-J. Romberg, A. Winkler, G. Dusel, E. Herzog, E. Most, K. Eder
Journal of Dairy Science.2015; 98(12): 8856. CrossRef - Vascular protection with fibroblast growth factor 21 in diabetes: Its potential beyond glucose and lipid control
Mi-Hua Liu
International Journal of Cardiology.2015; 199: 403. CrossRef
- Fibroblast growth factor 21: An emerging pleiotropic regulator of lipid metabolism and the metabolic network
- Pattern of Stress-Induced Hyperglycemia according to Type of Diabetes: A Predator Stress Model
- Jin-Sun Chang, Young-Hye You, Shin-Young Park, Ji-Won Kim, Hun-Sung Kim, Kun-Ho Yoon, Jae-Hyoung Cho
- Diabetes Metab J. 2013;37(6):475-483. Published online December 12, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.6.475
- 4,050 View
- 50 Download
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background We aimed to quantify stress-induced hyperglycemia and differentiate the glucose response between normal animals and those with diabetes. We also examined the pattern in glucose fluctuation induced by stress according to type of diabetes.
Methods To load psychological stress on animal models, we used a predator stress model by exposing rats to a cat for 60 minutes and measured glucose level from the beginning to the end of the test to monitor glucose fluctuation. We induced type 1 diabetes model (T1D) for ten Sprague-Dawley rats using streptozotocin and used five Otsuka Long-Evans Tokushima Fatty rats as obese type 2 diabetes model (OT2D) and 10 Goto-Kakizaki rats as nonobese type 2 diabetes model (NOT2D). We performed the stress loading test in both the normal and diabetic states and compared patterns of glucose fluctuation among the three models. We classified the pattern of glucose fluctuation into A, B, and C types according to speed of change in glucose level.
Results Increase in glucose, total amount of hyperglycemic exposure, time of stress-induced hyperglycemia, and speed of glucose increase were significantly increased in all models compared to the normal state. While the early increase in glucose after exposure to stress was higher in T1D and NOT2D, it was slower in OT2D. The rate of speed of the decrease in glucose level was highest in NOT2D and lowest in OT2D.
Conclusion The diabetic state was more vulnerable to stress compared to the normal state in all models, and the pattern of glucose fluctuation differed among the three types of diabetes. The study provides basic evidence for stress-induced hyperglycemia patterns and characteristics used for the management of diabetes patients.
-
Citations
Citations to this article as recorded by- Stress hyperglycemia as first sign of asymptomatic type 1 diabetes: an instructive case
Wei-De Wang, Chun-Hao Chu, Chiung-Hsi Tien, Shuo-Yu Wang, Shih-Yao Liu, Chien-Ming Lin
BMC Pediatrics.2021;[Epub] CrossRef - Genetic determinants of obesity heterogeneity in type II diabetes
Somayeh Alsadat Hosseini Khorami, Mohd Sokhini Abd Mutalib, Mohammad Feili Shiraz, Joseph Anthony Abdullah, Zulida Rejali, Razana Mohd Ali, Huzwah Khaza’ai
Nutrition & Metabolism.2020;[Epub] CrossRef - Sex Dimorphic Responses of the Hypothalamus–Pituitary–Thyroid Axis to Maternal Separation and Palatable Diet
Lorraine Jaimes-Hoy, Fidelia Romero, Jean-Louis Charli, Patricia Joseph-Bravo
Frontiers in Endocrinology.2019;[Epub] CrossRef - Hesperidin protects against stress induced gastric ulcer through regulation of peroxisome proliferator activator receptor gamma in diabetic rats
Shimaa M. Elshazly, Dalia M. Abd El Motteleb, Islam A.A.E-H. Ibrahim
Chemico-Biological Interactions.2018; 291: 153. CrossRef - Physiology and Neurobiology of Stress and the Implications for Physical Health
B Sivaprakash
Annals of SBV.2014; 3(1): 25. CrossRef
- Stress hyperglycemia as first sign of asymptomatic type 1 diabetes: an instructive case
- The Interplay between Autophagy and Aging
- Jong-Ok Pyo, Seung-Min Yoo, Yong-Keun Jung
- Diabetes Metab J. 2013;37(5):333-339. Published online October 17, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.5.333
- 3,786 View
- 37 Download
- 25 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Numerous studies have established a link between autophagy and aging; however, the relationship has not been clearly defined. Aging is a very complex process caused by the accumulation of various factors due to the gradual failure of cellular maintenance. Recent studies have shown that autophagy reduces the stress responses induced by starvation, reactive oxygen species, and the accumulation of intracellular proteins and organelles through cytoprotection, clearance of damaged mitochondria, and lysosomal degradation. Here, we summarize our current understanding of the relationship between autophagy and the aging process.
-
Citations
Citations to this article as recorded by- Dysregulation of autophagy activation induced by atorvastatin contributes to new-onset diabetes mellitus in western diet-fed mice
Juhee Kim, Minjune Kim, Minjeong Kim, Young-Hye You, Youngmi Song, Byung-Wan Lee
Metabolism.2024; 153: 155795. CrossRef - The impact of zinc on the molecular signaling pathways in the diabetes disease
Keyvan Asghari, Zahra Shargh, Sina Fatehfar, Leila Chodari, Parsa Sameei
Journal of Trace Elements in Medicine and Biology.2022; 72: 126985. CrossRef - Antiaging agents: safe interventions to slow aging and healthy life span extension
Ji-Kai Liu
Natural Products and Bioprospecting.2022;[Epub] CrossRef - Glycans in autophagy, endocytosis and lysosomal functions
Fulvio Reggiori, Hans-Joachim Gabius, Massimo Aureli, Winfried Römer, Sandro Sonnino, Eeva-Liisa Eskelinen
Glycoconjugate Journal.2021; 38(5): 625. CrossRef - Reduced cardiomyocyte Na+ current in the age‐dependent murine Pgc‐1β−/− model of ventricular arrhythmia
Shiraz Ahmad, Haseeb Valli, Robert Smyth, Anita Y. Jiang, Kamalan Jeevaratnam, Hugh R. Matthews, Christopher L.‐H. Huang
Journal of Cellular Physiology.2019; 234(4): 3921. CrossRef - Autophagy in Human Health and Disease: Novel Therapeutic Opportunities
Francesca Giampieri, Sadia Afrin, Tamara Y. Forbes-Hernandez, Massimiliano Gasparrini, Danila Cianciosi, Patricia Reboredo-Rodriguez, Alfonso Varela-Lopez, Jose L. Quiles, Maurizio Battino
Antioxidants & Redox Signaling.2019; 30(4): 577. CrossRef - Molybdenum Disulfide Nanoparticles Resist Oxidative Stress-Mediated Impairment of Autophagic Flux and Mitigate Endothelial Cell Senescence and Angiogenic Dysfunctions
Sunkui Ke, Youlin Lai, Tong Zhou, Lihuang Li, Yange Wang, Lei Ren, Shefang Ye
ACS Biomaterials Science & Engineering.2018; 4(2): 663. CrossRef - Phenformin inhibits cell proliferation and induces cell apoptosis and autophagy in cholangiocarcinoma
Shuyang Hu, Qing Ouyang, Qingbao Cheng, Jinghan Wang, Feiling Feng, Liang Qiao, Wei Gan, Yang Shi, Demin Wu, Xiaoqing Jiang
Molecular Medicine Reports.2018;[Epub] CrossRef - Ventricular pro-arrhythmic phenotype, arrhythmic substrate, ageing and mitochondrial dysfunction in peroxisome proliferator activated receptor-γ coactivator-1β deficient (Pgc-1β) murine hearts
Shiraz Ahmad, Haseeb Valli, Karan R. Chadda, James Cranley, Kamalan Jeevaratnam, Christopher L.-H. Huang
Mechanisms of Ageing and Development.2018; 173: 92. CrossRef - From Christian de Duve to Yoshinori Ohsumi: More to autophagy than just dining at home
Margaret M. Harnett, Miguel A. Pineda, Perle Latré de Laté, Russell J. Eason, Sébastien Besteiro, William Harnett, Gordon Langsley
Biomedical Journal.2017; 40(1): 9. CrossRef - Metformin Restores Parkin-Mediated Mitophagy, Suppressed by Cytosolic p53
Young Song, Woo Lee, Yong-ho Lee, Eun Kang, Bong-Soo Cha, Byung-Wan Lee
International Journal of Molecular Sciences.2016; 17(1): 122. CrossRef - Selected reaction monitoring mass spectrometry for relative quantification of proteins involved in cellular life and death processes
Rune Isak Dupont Birkler, Zahra Nochi, Niels Gregersen, Johan Palmfeldt
Journal of Chromatography B.2016; 1035: 49. CrossRef - Dehydroepiandrosterone prevents linoleic acid-induced endothelial cell senescence by increasing autophagy
Min Jung Lee, Eun Hee Kim, Sang Ah. Lee, Yu Mi Kang, Chang Hee Jung, Hae Kyeong Yoon, So Mi Seol, Yoo La Lee, Woo Je Lee, Joong-Yeol Park
Metabolism.2015; 64(9): 1134. CrossRef - Successful aging: Advancing the science of physical independence in older adults
Stephen D. Anton, Adam J. Woods, Tetso Ashizawa, Diana Barb, Thomas W. Buford, Christy S. Carter, David J. Clark, Ronald A. Cohen, Duane B. Corbett, Yenisel Cruz-Almeida, Vonetta Dotson, Natalie Ebner, Philip A. Efron, Roger B. Fillingim, Thomas C. Foster
Ageing Research Reviews.2015; 24: 304. CrossRef - Autophagy in acute leukemias: A double-edged sword with important therapeutic implications
Cecilia Evangelisti, Camilla Evangelisti, Francesca Chiarini, Annalisa Lonetti, Francesca Buontempo, Luca M. Neri, James A. McCubrey, Alberto M. Martelli
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research.2015; 1853(1): 14. CrossRef - Autophagy: A housekeeper in cardiorenal metabolic health and disease
Guanghong Jia, James R. Sowers
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2015; 1852(2): 219. CrossRef - Autophagy and Lipid Droplets in the Liver
Nuria Martinez-Lopez, Rajat Singh
Annual Review of Nutrition.2015; 35(1): 215. CrossRef - Lysosomal Two-pore Channel Subtype 2 (TPC2) Regulates Skeletal Muscle Autophagic Signaling
Pei-Hui Lin, Pu Duann, Shinji Komazaki, Ki Ho Park, Haichang Li, Mingzhai Sun, Mathew Sermersheim, Kristyn Gumpper, John Parrington, Antony Galione, A. Mark Evans, Michael X. Zhu, Jianjie Ma
Journal of Biological Chemistry.2015; 290(6): 3377. CrossRef - Zinc and autophagy
Juan P. Liuzzi, Liang Guo, Changwon Yoo, Tiffanie S. Stewart
BioMetals.2014; 27(6): 1087. CrossRef - Autophagy in the eye: Implications for ocular cell health
Laura S. Frost, Claire H. Mitchell, Kathleen Boesze-Battaglia
Experimental Eye Research.2014; 124: 56. CrossRef - Can Enhanced Autophagy Be Associated with Human Longevity? Serum Levels of the Autophagy Biomarker Beclin-1 Are Increased in Healthy Centenarians
Enzo Emanuele, Piercarlo Minoretti, Fabian Sanchis-Gomar, Helios Pareja-Galeano, Yusuf Yilmaz, Nuria Garatachea, Alejandro Lucia
Rejuvenation Research.2014; 17(6): 518. CrossRef - Responses and adaptations of intervertebral disc cells to microenvironmental stress: a possible central role of autophagy in the adaptive mechanism
Libo Jiang, Fenglai Yuan, Xiaofan Yin, Jian Dong
Connective Tissue Research.2014; 55(5-6): 311. CrossRef - STOP accelerating lung aging for the treatment of COPD
Kazuhiro Ito, Nicolas Mercado
Experimental Gerontology.2014; 59: 21. CrossRef - In search of antiaging modalities: Evaluation of mTOR‐ and ROS/DNA damage‐signaling by cytometry
Zbigniew Darzynkiewicz, Hong Zhao, H. Dorota Halicka, Jiangwei Li, Yong‐Syu Lee, Tze‐Chen Hsieh, Joseph M. Wu
Cytometry Part A.2014; 85(5): 386. CrossRef - Induction of Covalently Crosslinked p62 Oligomers with Reduced Binding to Polyubiquitinated Proteins by the Autophagy Inhibitor Verteporfin
Elizabeth Donohue, Aruna D. Balgi, Masaaki Komatsu, Michel Roberge, Srinivasa M. Srinivasula
PLoS ONE.2014; 9(12): e114964. CrossRef
- Dysregulation of autophagy activation induced by atorvastatin contributes to new-onset diabetes mellitus in western diet-fed mice
- The Role of Oxidative Stress in the Pathogenesis of Diabetic Vascular Complications
- Shuji Sasaki, Toyoshi Inoguchi
- Diabetes Metab J. 2012;36(4):255-261. Published online August 20, 2012
- DOI: https://doi.org/10.4093/dmj.2012.36.4.255
- 3,793 View
- 61 Download
- 60 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Oxidative stress has been paid increasing attention to as an important causative factor for diabetic vascular complications. Among possible various sources, accumulating evidence has indicated that NAD(P)H oxidase may be the most important source for reactive oxygen species production in diabetic vascular tissues. The mechanisms underlying activation and up-regulation of NAD(P)H oxidase has been supposed to be mediated by high glucose-induced protein kinase C (PKC) activation. In this review article, activation of local renin-angiotensin II system induced by chymase activation is also shown to amplify such a PKC-dependent activation of NAD(P)H oxidase. Additionally, human evidence showing the beneficial effect of antioxidants on diabetic vascular complications. Bilirubin has been recognized as a strong endogenous antioxidant. Here markedly lower prevalence of vascular complications is shown in diabetic patients with Gilbert syndrome, a congenital hyperbilirubinemia, as well as reduced markers of oxidative stress and inflammation. Lastly, statin, angiotensin II receptor blocker, chymase inhibitor, bilirubin and biliverdin, PKC β isoform inhibitor, and glucagon-like peptide-1 analog, are shown to serve as antioxidants and have some beneficial effect on diabetic vascular complications, via inhibiting PKC-NAD(P)H oxidase activation, supporting the notion that this mechanism may be an effective therapeutic target for preventing diabetic vascular complications.
-
Citations
Citations to this article as recorded by- The effects of whey protein on anthropometric parameters, resting energy expenditure, oxidative stress, and appetite in overweight/obese women with type 2 diabetes mellitus: A randomized placebo controlled clinical trial
Maryam Nouri, Bahram Pourghassem Gargari, Zahra Ghasempour, Vahideh Sadra, Mohammad Asghari Jafarabadi, Arvin Babaei, Pedram Tajfar, Ali Tarighat-Esfanjani
International Journal of Diabetes in Developing Countries.2024; 44(1): 155. CrossRef - Rhodanine-benzamides as potential hits for α-amylase enzyme inhibitors and radical (DPPH and ABTS) scavengers
Samuel Attah Egu, Irfan Ali, Khalid Mohammed Khan, Sridevi Chigurupati, Urooj Qureshi, Uzma Salar, Zaheer Ul-Haq, Suliman A. Almahmoud, Shatha Ghazi Felemban, Mohsin Ali, Muhammad Taha
Molecular Diversity.2024;[Epub] CrossRef - Research Progress of FGF-21 and CVAI in Type 2 Diabetic Kidney Disease
央宗 才巴
Advances in Clinical Medicine.2023; 13(07): 12056. CrossRef - Is Subcutaneous Rifamycin Application Superior to Saline Application in Hip Hemiarthroplasty?
Malik Çelik, Nezih Ziroğlu, Alkan Bayrak
Bakirkoy Tip Dergisi / Medical Journal of Bakirkoy.2023; 19(2): 186. CrossRef - In pursuit of incorporation of markers of oxidative stress in traditional biochemical panels in clinical Chemistry: A risk assessment step in diagnosis and biotherapy
John Ibhagbemien Anetor, Chukwuemelie Zedech Uche, Gloria Oiyahumen Anetor
American Journal of Biopharmacy and Pharmaceutical Sciences.2022; 2: 1. CrossRef - Pathophysiologic Mechanisms and Potential Biomarkers in Diabetic Kidney Disease
Chan-Young Jung, Tae-Hyun Yoo
Diabetes & Metabolism Journal.2022; 46(2): 181. CrossRef - p38 MAPK Inhibitor (SB203580) and Metformin Reduces Aortic Protein Carbonyl and Inflammation in Non-obese Type 2 Diabetic Rats
Nuttikarn Nokkaew, Podsawee Mongkolpathumrat, Ruttanapong Junsiri, Supawit Jindaluang, Nichagron Tualamun, Niya Manphatthanakan, Nareumon Saleesee, Marisa Intasang, Jantira Sanit, Punyanuch Adulyaritthikul, Kantapich Kongpol, Sarawut Kumphune, Nitirut Ner
Indian Journal of Clinical Biochemistry.2021; 36(2): 228. CrossRef - Resveratrol-Elicited PKC Inhibition Counteracts NOX-Mediated Endothelial to Mesenchymal Transition in Human Retinal Endothelial Cells Exposed to High Glucose
Roberta Giordo, Gheyath K. Nasrallah, Anna Maria Posadino, Francesco Galimi, Giampiero Capobianco, Ali Hussein Eid, Gianfranco Pintus
Antioxidants.2021; 10(2): 224. CrossRef - BIOLOGICAL ACTIVE COMPONENTS OF SILENE TOMENTELLA AND THEIR PHARMACOLOGICAL PROPERTIES
Ugiloy Yusufovna Yusupova, Nurmurod Sheralievich Ramazonov, Khairulla Mamadievich Bobakulov, Feruza Rustamovna Egamova, Vladimir Nikolaevich Syrov, Durbek Abdikhoshimovich Usmanov
chemistry of plant raw material.2021; (1): 197. CrossRef - Chronically Elevated Exogenous Glucose Elicits Antipodal Effects on the Proteome Signature of Differentiating Human iPSC-Derived Pancreatic Progenitors
Luiza Ghila, Thomas Aga Legøy, Andreas Frøslev Mathisen, Shadab Abadpour, Joao A. Paulo, Hanne Scholz, Helge Ræder, Simona Chera
International Journal of Molecular Sciences.2021; 22(7): 3698. CrossRef - Effect of Type-2 Diabetes Mellitus in Retinopathy Patients on MDA, SOD Activity and its Correlation with HbA1c
Yali Hou, Mei Lin, Xuan Qiu, Mingjuan He, Yu Zhang, Feifei Guo
Brazilian Archives of Biology and Technology.2021;[Epub] CrossRef - Ameliorative effects of Hydrolea zeylanica in streptozotocin-induced oxidative stress and metabolic changes in diabetic rats
Sandeep Kumar Swain, Umesh Chandra Dash, Satish Kanhar, Atish Kumar Sahoo
Journal of Ethnopharmacology.2020; 247: 112257. CrossRef - Imbalance in the antioxidant defence system and pro-genotoxic status induced by high glucose concentrations: In vitro testing in human liver cells
Mattia Acito, Desirée Bartolini, Maria Rachele Ceccarini, Carla Russo, Samuele Vannini, Luca Dominici, Michela Codini, Milena Villarini, Francesco Galli, Tommaso Beccari, Massimo Moretti
Toxicology in Vitro.2020; 69: 105001. CrossRef - Synthesis of sulpha drug based hydroxytriazene derivatives: Anti-diabetic, antioxidant, anti-inflammatory activity and their molecular docking studies
Poonam Sharma, Varsha Dayma, Aparna Dwivedi, Prabhat K. Baroliya, I.P. Tripathi, Murugesan Vanangamudi, R.S. Chauhan, A.K. Goswami
Bioorganic Chemistry.2020; 96: 103642. CrossRef - Analysis of long non-coding RNA expression profiles in high-glucose treated vascular endothelial cells
Erqin Xu, Xiaolei Hu, Xiaoli Li, Guoxi Jin, Langen Zhuang, Qiong Wang, Xiaoyan Pei
BMC Endocrine Disorders.2020;[Epub] CrossRef - Potent α-amylase inhibitors and radical (DPPH and ABTS) scavengers based on benzofuran-2-yl(phenyl)methanone derivatives: Syntheses, in vitro, kinetics, and in silico studies
Irfan Ali, Rafaila Rafique, Khalid Mohammed Khan, Sridevi Chigurupati, Xingyue Ji, Abdul Wadood, Ashfaq Ur Rehman, Uzma Salar, Muhammad Shahid Iqbal, Muhammad Taha, Shahnaz Perveen, Basharat Ali
Bioorganic Chemistry.2020; 104: 104238. CrossRef - A review of fibroblast growth factor 21 in diabetic cardiomyopathy
Xiang Zhang, Luo Yang, Xiongfeng Xu, Fengjuan Tang, Peng Yi, Bo Qiu, Yarong Hao
Heart Failure Reviews.2019; 24(6): 1005. CrossRef - Recent advances in the pathogenesis of microvascular complications in diabetes
Sungmi Park, Hyeon-Ji Kang, Jae-Han Jeon, Min-Ji Kim, In-Kyu Lee
Archives of Pharmacal Research.2019; 42(3): 252. CrossRef - Metabolic syndrome status over 2 years predicts incident chronic kidney disease in mid-life adults: a 10-year prospective cohort study
So Jin Lee, Hun Ju Lee, Hyun jeong Oh, Taehwa Go, Dae Ryong Kang, Jang Young Kim, Ji Hye Huh
Scientific Reports.2018;[Epub] CrossRef - Role of peroxisome proliferator–activated receptor-gamma activation on visfatin, advanced glycation end products, and renal oxidative stress in obesity-induced type 2 diabetes mellitus
A Tabassum, T Mahboob
Human & Experimental Toxicology.2018; 37(11): 1187. CrossRef - Coffee consumption and reduced risk of developing type 2 diabetes: a systematic review with meta-analysis
Mattias Carlström, Susanna C Larsson
Nutrition Reviews.2018; 76(6): 395. CrossRef - Liraglutide ameliorates early renal injury by the activation of renal FoxO1 in a type 2 diabetic kidney disease rat model
Pin Chen, Xiaozhi Shi, Xiangjin Xu, Yiyang Lin, Zhulin Shao, Rongdan Wu, Lihong Huang
Diabetes Research and Clinical Practice.2018; 137: 173. CrossRef - Antioxidant effects of vitamins in type 2 diabetes: a meta-analysis of randomized controlled trials
Maria E. Balbi, Fernanda S. Tonin, Antonio M. Mendes, Helena H. Borba, Astrid Wiens, Fernando Fernandez-Llimos, Roberto Pontarolo
Diabetology & Metabolic Syndrome.2018;[Epub] CrossRef - Gilbert syndrome in patients with type 1 diabetes-Prevalence, glycemic control, and microalbuminuria
Sigal Singer, Nurit Pilpel, Orit Pinhas-Hamiel
Pediatric Diabetes.2017; 18(8): 803. CrossRef - Low serum bilirubin level predicts the development of chronic kidney disease in patients with type 2 diabetes mellitus
Kang Hee Ahn, Sang Soo Kim, Won Jin Kim, Jong Ho Kim, Yun Jeong Nam, Su Bin Park, Yun Kyung Jeon, Bo Hyun Kim, In Joo Kim, Yong Ki Kim
The Korean Journal of Internal Medicine.2017; 32(5): 875. CrossRef - Tiron ameliorates high glucose‐induced cardiac myocyte apoptosis by PKCδ‐dependent inhibition of osteopontin
Ping Jiang, Deling Zhang, Hong Qiu, Xianqi Yi, Yemin Zhang, Yingkang Cao, Bo Zhao, Zhongyuan Xia, Changhua Wang
Clinical and Experimental Pharmacology and Physiology.2017; 44(7): 760. CrossRef - Protective effects of 6-Gingerol on vascular endothelial cell injury induced by high glucose via activation of PI3K-AKT-eNOS pathway in human umbilical vein endothelial cells
Dan Liu, Mengqing Wu, Yi Lu, Tao Xian, Yupeng Wang, Bowei Huang, Guohua Zeng, Qiren Huang
Biomedicine & Pharmacotherapy.2017; 93: 788. CrossRef - Adenosine signaling in diabetes mellitus and associated cardiovascular and renal complications
Maria Peleli, Mattias Carlstrom
Molecular Aspects of Medicine.2017; 55: 62. CrossRef - Diabetes and increased lipid peroxidation are associated with systemic inflammation even in well-controlled patients
Alliny de Souza Bastos, Dana T. Graves, Ana Paula de Melo Loureiro, Carlos Rossa Júnior, Sâmia Cruz Tfaile Corbi, Fausto Frizzera, Raquel Mantuaneli Scarel-Caminaga, Niels Olsen Câmara, Oelisoa M. Andriankaja, Meire I. Hiyane, Silvana Regina Perez Orrico
Journal of Diabetes and its Complications.2016; 30(8): 1593. CrossRef - Agkistrodon acutus-purified protein C activator protects human umbilical vein endothelial cells against H2O2-induced apoptosis
Shu Li, Yun Hong, Xin Jin, Xianwei Li, Entao Sun, Genbao Zhang, Linming Lu, Liuwang Nie
Pharmaceutical Biology.2016; 54(12): 3285. CrossRef - The impact of mast cells on cardiovascular diseases
Eva Kritikou, Johan Kuiper, Petri T. Kovanen, Ilze Bot
European Journal of Pharmacology.2016; 778: 103. CrossRef - Comparison of acarbose and metformin therapy in newly diagnosed type 2 diabetic patients with overweight and/or obesity
Weiping Sun, Chunping Zeng, Lizhen Liao, Juan Chen, Ying Wang
Current Medical Research and Opinion.2016; 32(8): 1389. CrossRef - Mechanisms for the cardiovascular effects of glucagon-like peptide-1
H. Poudyal
Acta Physiologica.2016; 216(3): 277. CrossRef - Integrated scientific data bases review on asulacrine and associated toxicity
Attia Afzal, Muhammad Sarfraz, Zimei Wu, Guangji Wang, Jianguo Sun
Critical Reviews in Oncology/Hematology.2016; 104: 78. CrossRef - Impact of Diabetes and Defective Thyroid Ontogenesis on Audition
Luciene da Cruz Fernandes
Journal of Diabetes, Metabolic Disorders & Control.2016;[Epub] CrossRef - Serum Total Bilirubin Levels Provide Additive Risk Information over the Framingham Risk Score for Identifying Asymptomatic Diabetic Patients at Higher Risk for Coronary Artery Stenosis
Jaechan Leem, Eun Hee Koh, Jung Eun Jang, Chang-Yun Woo, Jin Sun Oh, Min Jung Lee, Joon-Won Kang, Tae-Hwan Lim, Chang Hee Jung, Woo Je Lee, Joong-Yeol Park, Ki-Up Lee
Diabetes & Metabolism Journal.2015; 39(5): 414. CrossRef - Clinical implications of carotid artery intima media thickness assessment on cardiovascular risk stratification in hyperlipidemic Korean adults with diabetes: the ALTO study
Eun-Gyoung Hong, Jung Hun Ohn, Seong Jin Lee, Hyuk Sang Kwon, Sin Gon Kim, Dong Jun Kim, Dong Sun Kim
BMC Cardiovascular Disorders.2015;[Epub] CrossRef - Undiagnosed diabetes is prevalent in younger adults and associated with a higher risk cardiometabolic profile compared to diagnosed diabetes
Yong-ho Lee, Ehrin J. Armstrong, Gyuri Kim, Jaewon Oh, Seok-Min Kang, Byung-Wan Lee, Chul Woo Ahn, Bong Soo Cha, Hyun Chul Lee, Christos S. Mantzoros, Eun Seok Kang
American Heart Journal.2015; 170(4): 760. CrossRef - Common polymorphisms in antioxidant genes are associated with diabetic nephropathy in Type 2 diabetes patients
Jasna Klen, Katja Goričar, Andrej Janež, Vita Dolžan
Personalized Medicine.2015; 12(3): 187. CrossRef - Increased endogenous serotonin level in diabetic conditions may lead to cardiac valvulopathy via reactive oxygen species regulation
Tahir Ali, Hina Waheed, Farhat Shaheen, Madiha Mahmud, Qamar Javed, Iram Murtaza
Biologia.2015; 70(2): 273. CrossRef - Antioxidant Effects of Statins in the Management of Cardiometabolic Disorders
Soo Lim, Philip Barter
Journal of Atherosclerosis and Thrombosis.2014; 21(10): 997. CrossRef - Evidence-Based Practice Use of Incretin-Based Therapy in the Natural History of Diabetes
Stanley Schwartz
Postgraduate Medicine.2014; 126(3): 66. CrossRef - Low serum bilirubin concentration is a novel risk factor for the development of albuminuria in patients with type 2 diabetes
Hiroshi Okada, Michiaki Fukui, Muhei Tanaka, Shinobu Matsumoto, Kanae Kobayashi, Hiroya Iwase, Kiichiro Tomiyasu, Koji Nakano, Goji Hasegawa, Naoto Nakamura
Metabolism.2014; 63(3): 409. CrossRef - Serum total bilirubin levels and prevalence of diabetic retinopathy in a Chinese population (中国人群血清总胆红素水平与糖尿病视网膜病变相关性的研究)
Syeda Sadia Najam, Jichao Sun, Jie Zhang, Min Xu, Jieli Lu, Kan Sun, Mian Li, Tiange Wang, Yufang Bi, Guang Ning
Journal of Diabetes.2014; 6(3): 221. CrossRef - Roles of oxidative stress, adiponectin, and nuclear hormone receptors in obesity-associated insulin resistance and cardiovascular risk
Morihiro Matsuda, Iichiro Shimomura
Hormone Molecular Biology and Clinical Investigation.2014;[Epub] CrossRef - Unilateral injection of Aβ25–35 in the hippocampus reduces the number of dendritic spines in hyperglycemic rats
Zayda Lazcano, Oscar Solis, María Elena Bringas, Daniel Limón, Alfonso Diaz, Blanca Espinosa, Isabel García‐Peláez, Gonzalo Flores, Jorge Guevara
Synapse.2014; 68(12): 585. CrossRef - Role of angiotensin-converting enzyme 2 (ACE2) in diabetic cardiovascular complications
Vaibhav B. Patel, Nirmal Parajuli, Gavin Y. Oudit
Clinical Science.2014; 126(7): 471. CrossRef - Pathogenesis of Chronic Hyperglycemia: From Reductive Stress to Oxidative Stress
Liang-Jun Yan
Journal of Diabetes Research.2014; 2014: 1. CrossRef - Daily intake of vitamin D‐ or calcium‐vitamin D‐fortified Persian yogurt drink (doogh) attenuates diabetes‐induced oxidative stress: evidence for antioxidative properties of vitamin D
B. Nikooyeh, T. R. Neyestani, N. Tayebinejad, H. Alavi‐Majd, N. Shariatzadeh, A. Kalayi, M. Zahedirad, S. Heravifard, S. Salekzamani
Journal of Human Nutrition and Dietetics.2014; 27(s2): 276. CrossRef - Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases
Morihiro Matsuda, Iichiro Shimomura
Reviews in Endocrine and Metabolic Disorders.2014; 15(1): 1. CrossRef - PKCδ-dependent Activation of the Ubiquitin Proteasome System is Responsible for High Glucose-induced Human Breast Cancer MCF-7 Cell Proliferation, Migration and Invasion
Shan Zhu, Feng Yao, Wen-Huan Li, Jin-Nan Wan, Yi-Min Zhang, Zhao Tang, Shahzad Khan, Chang-Hua Wang, Sheng-Rong Sun
Asian Pacific Journal of Cancer Prevention.2013; 14(10): 5687. CrossRef - Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer
Morihiro Matsuda, Iichiro Shimomura
Obesity Research & Clinical Practice.2013; 7(5): e330. CrossRef - Hydrogen Sulfide Suppresses High Glucose–Induced Expression of Intercellular Adhesion Molecule-1 in Endothelial Cells
Qingbo Guan, Xiaolei Wang, Ling Gao, Jicui Chen, Yuantao Liu, Chunxiao Yu, Nan Zhang, Xu Zhang, Jiajun Zhao
Journal of Cardiovascular Pharmacology.2013; 62(3): 278. CrossRef - Clinical Marker of Platelet Hyperreactivity in Diabetes Mellitus
Jin Hwa Kim, Hak Yeon Bae, Sang Yong Kim
Diabetes & Metabolism Journal.2013; 37(6): 423. CrossRef - Glucose initially inhibits and later stimulates blood ROS generation
Thomas Stief
Journal of Diabetes Mellitus.2013; 03(01): 15. CrossRef - Oxidative Stress in Cardiovascular Diseases and Obesity: Role of p66Shc and Protein Kinase C
Elena De Marchi, Federica Baldassari, Angela Bononi, Mariusz R. Wieckowski, Paolo Pinton
Oxidative Medicine and Cellular Longevity.2013; 2013: 1. CrossRef - Toxicological and pharmacological concerns on oxidative stress and related diseases
Soodabeh Saeidnia, Mohammad Abdollahi
Toxicology and Applied Pharmacology.2013; 273(3): 442. CrossRef - Comparison of antioxidant, α-glucosidase inhibition and anti-inflammatory activities of the leaf and root extracts ofSmilax chinaL.
Kyoung Kon Kim, Yun Hwan Kang, Dae Jung Kim, Tae Woo Kim, Myeon Choe
Journal of Nutrition and Health.2013; 46(4): 315. CrossRef - Correlation between blood glucose fluctuations and activation of oxidative stress in type 1 diabetic children during the acute metabolic disturbance period
Di WU, Chun-xiu GONG, Xi MENG, Qiu-lan YANG
Chinese Medical Journal.2013; 126(21): 4019. CrossRef - Oxidative stress and its complications in human health
Guniz M. Koksal
Advances in Bioscience and Biotechnology.2012; 03(08): 1113. CrossRef
- The effects of whey protein on anthropometric parameters, resting energy expenditure, oxidative stress, and appetite in overweight/obese women with type 2 diabetes mellitus: A randomized placebo controlled clinical trial

 KDA
KDA
 First
First Prev
Prev





