
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Mitochondrial Stress and Mitokines: Therapeutic Perspectives for the Treatment of Metabolic Diseases
- Benyuan Zhang, Joon Young Chang, Min Hee Lee, Sang-Hyeon Ju, Hyon-Seung Yi, Minho Shong
- Diabetes Metab J. 2024;48(1):1-18. Published online January 3, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0115
- 2,118 View
- 258 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
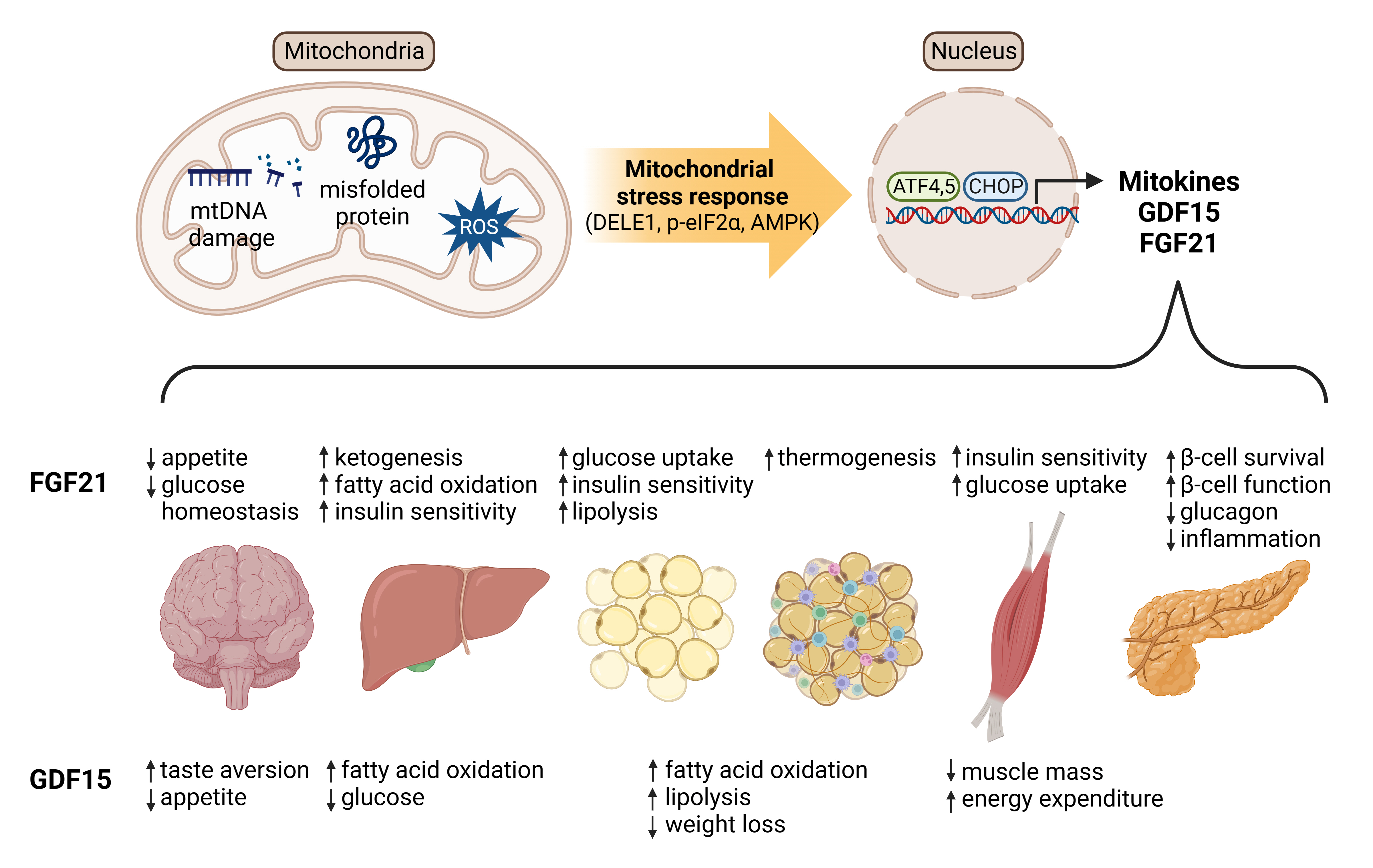
ePub - Mitochondrial stress and the dysregulated mitochondrial unfolded protein response (UPRmt) are linked to various diseases, including metabolic disorders, neurodegenerative diseases, and cancer. Mitokines, signaling molecules released by mitochondrial stress response and UPRmt, are crucial mediators of inter-organ communication and influence systemic metabolic and physiological processes. In this review, we provide a comprehensive overview of mitokines, including their regulation by exercise and lifestyle interventions and their implications for various diseases. The endocrine actions of mitokines related to mitochondrial stress and adaptations are highlighted, specifically the broad functions of fibroblast growth factor 21 and growth differentiation factor 15, as well as their specific actions in regulating inter-tissue communication and metabolic homeostasis. Finally, we discuss the potential of physiological and genetic interventions to reduce the hazards associated with dysregulated mitokine signaling and preserve an equilibrium in mitochondrial stress-induced responses. This review provides valuable insights into the mechanisms underlying mitochondrial regulation of health and disease by exploring mitokine interactions and their regulation, which will facilitate the development of targeted therapies and personalized interventions to improve health outcomes and quality of life.
- Pathophysiology
- Endoplasmic Reticulum Stress and Dysregulated Autophagy in Human Pancreatic Beta Cells
- Seoil Moon, Hye Seung Jung
- Diabetes Metab J. 2022;46(4):533-542. Published online July 27, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0070
- 4,573 View
- 250 Download
- 8 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
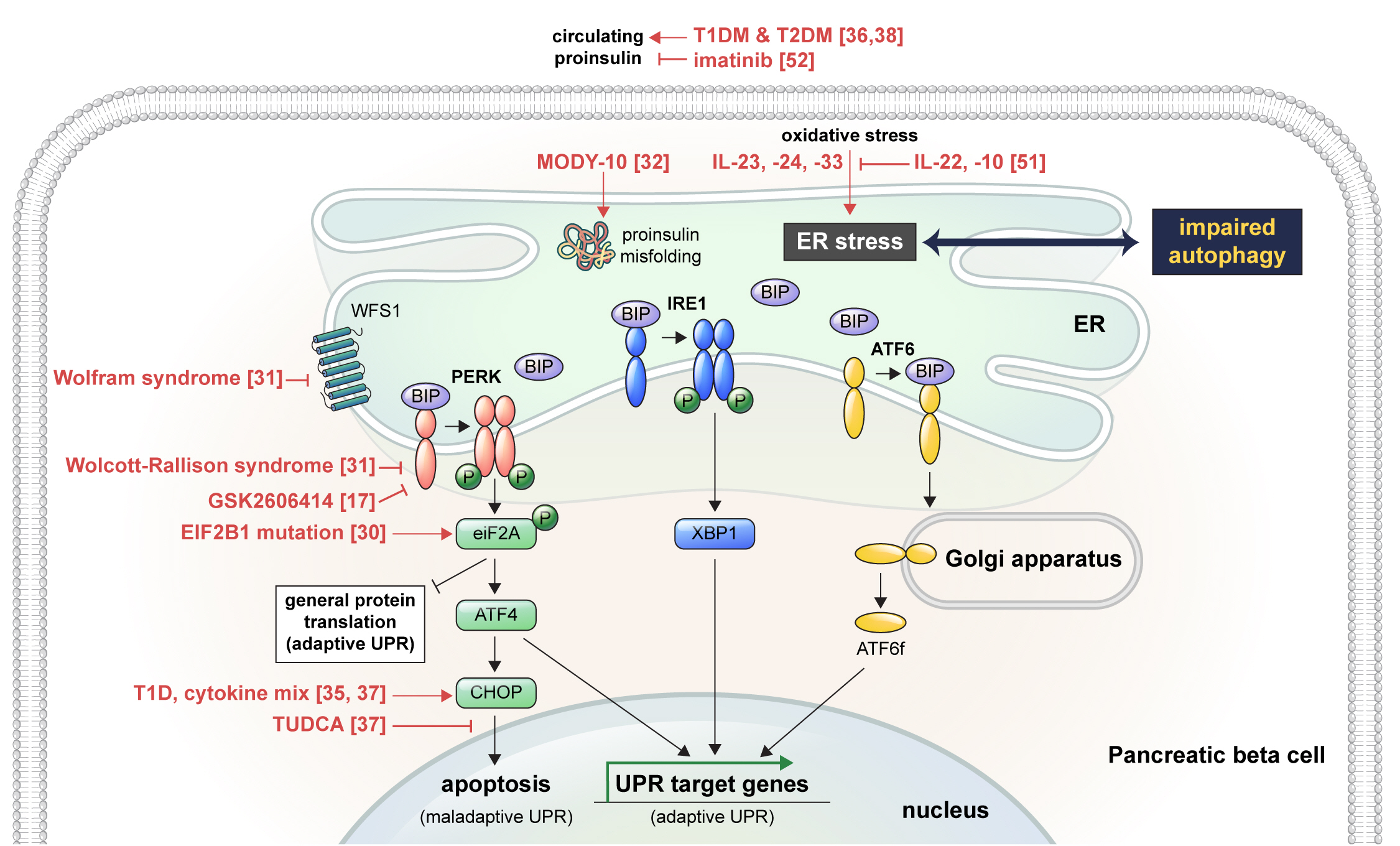
ePub - Pancreatic beta cell homeostasis is crucial for the synthesis and secretion of insulin; disruption of homeostasis causes diabetes, and is a treatment target. Adaptation to endoplasmic reticulum (ER) stress through the unfolded protein response (UPR) and adequate regulation of autophagy, which are closely linked, play essential roles in this homeostasis. In diabetes, the UPR and autophagy are dysregulated, which leads to beta cell failure and death. Various studies have explored methods to preserve pancreatic beta cell function and mass by relieving ER stress and regulating autophagic activity. To promote clinical translation of these research results to potential therapeutics for diabetes, we summarize the current knowledge on ER stress and autophagy in human insulin-secreting cells.
-
Citations
Citations to this article as recorded by- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
Seoil Moon, Ji Yoon Lim, Mirang Lee, Youngmin Han, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Mi Na Kim, Kyong Soo Park, Hye Seung Jung
Diabetes & Metabolism Journal.2024; 48(2): 231. CrossRef - Endoplasmic reticulum stress: A possible connection between intestinal inflammation and neurodegenerative disorders
Giorgio Vivacqua, Romina Mancinelli, Stefano Leone, Rosa Vaccaro, Ludovica Garro, Simone Carotti, Ludovica Ceci, Paolo Onori, Luigi Pannarale, Antonio Franchitto, Eugenio Gaudio, Arianna Casini
Neurogastroenterology & Motility.2024;[Epub] CrossRef - Docosahexanoic Acid Attenuates Palmitate-Induced Apoptosis by Autophagy Upregulation via GPR120/mTOR Axis in Insulin-Secreting Cells
Seok-Woo Hong, Jinmi Lee, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2024; 39(2): 353. CrossRef - Pancreatic islet remodeling in cotadutide-treated obese mice
Renata Spezani, Thatiany Souza Marinho, Luiz E. Macedo Cardoso, Marcia Barbosa Aguila, Carlos Alberto Mandarim-de-Lacerda
Life Sciences.2023; 327: 121858. CrossRef - Modulation of Unfolded Protein Response Restores Survival and Function of β-Cells Exposed to the Endocrine Disruptor Bisphenol A
Laura Maria Daian, Gabriela Tanko, Andrei Mircea Vacaru, Luiza Ghila, Simona Chera, Ana-Maria Vacaru
International Journal of Molecular Sciences.2023; 24(3): 2023. CrossRef - Interplay of skeletal muscle and adipose tissue: sarcopenic obesity
Min Jeong Park, Kyung Mook Choi
Metabolism.2023; 144: 155577. CrossRef - Identification and analysis of type 2 diabetes-mellitus-associated autophagy-related genes
Kun Cui, Zhizheng Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Sestrin2 in diabetes and diabetic complications
Xiaodan Zhang, Zirui Luo, Jiahong Li, Yaxuan Lin, Yu Li, Wangen Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Crosstalk between autophagy and insulin resistance: evidence from different tissues
Asie Sadeghi, Maryam Niknam, Mohammad Amin Momeni-Moghaddam, Maryam Shabani, Hamid Aria, Alireza Bastin, Maryam Teimouri, Reza Meshkani, Hamed Akbari
European Journal of Medical Research.2023;[Epub] CrossRef - Beta cell lipotoxicity in the development of type 2 diabetes: the need for species-specific understanding
Patricia Thomas, Meurig T. Gallagher, Gabriela Da Silva Xavier
Frontiers in Endocrinology.2023;[Epub] CrossRef
- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
- Pathophysiology
- Nuclear Receptors Resolve Endoplasmic Reticulum Stress to Improve Hepatic Insulin Resistance
- Jae Man Lee
- Diabetes Metab J. 2017;41(1):10-19. Published online February 16, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.1.10
- 4,616 View
- 94 Download
- 13 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Chronic endoplasmic reticulum (ER) stress culminating in proteotoxicity contributes to the development of insulin resistance and progression to type 2 diabetes mellitus. Pharmacologic interventions targeting several different nuclear receptors have emerged as potential treatments for insulin resistance. The mechanistic basis for these antidiabetic effects has primarily been attributed to multiple metabolic and inflammatory functions. Here we review recent advances in our understanding of the association of ER stress with insulin resistance and the role of nuclear receptors in promoting ER stress resolution and improving insulin resistance in the liver.
-
Citations
Citations to this article as recorded by- Duality of Nrf2 in iron-overload cardiomyopathy
Enrica Federti, Francesca Vinchi, Iana Iatcenko, Alessandra Ghigo, Alessandro Matte, Serge Cedrick Mbiandjeu Toya, Angela Siciliano, Deborah Chiabrando, Emanuela Tolosano, Steven Zebulon Vance, Veronica Riccardi, Immacolata Andolfo, Manuela Iezzi, Alessia
Haematologica.2023; 108(5): 1335. CrossRef - Endoplasmic Reticulum Stress and Its Impact on Adipogenesis: Molecular Mechanisms Implicated
Gyuhui Kim, Jiyoon Lee, Joohun Ha, Insug Kang, Wonchae Choe
Nutrients.2023; 15(24): 5082. CrossRef - Qingluotongbi formula regulates the LXRα-ERS-SREBP-1c pathway in hepatocytes to alleviate the liver injury caused by Tripterygium wilfordii Hook. f.
Zhichao Yu, Zhe Feng, Ling Fu, Jing Wang, Changqing Li, Huaxu Zhu, Tong Xie, Jie Zhou, Lingling Zhou, Xueping Zhou
Journal of Ethnopharmacology.2022; 287: 114952. CrossRef - Nuclear‐mitochondrial crosstalk: On the role of the nuclear receptor liver receptor homolog‐1 (NR5A2) in the regulation of mitochondrial metabolism, cell survival, and cancer
Svenja Michalek, Thomas Brunner
IUBMB Life.2021; 73(3): 592. CrossRef - NGBR is required to ameliorate type 2 diabetes in mice by enhancing insulin sensitivity
Yi Chen, Wenquan Hu, Qi Li, Shiwei Zhao, Dan Zhao, Shuang Zhang, Zhuo Wei, Xiaoxiao Yang, Yuanli Chen, Xiaoju Li, Chenzhong Liao, Jihong Han, Qing Robert Miao, Yajun Duan
Journal of Biological Chemistry.2021; 296: 100624. CrossRef - Impaired ferritinophagy flux induced by high fat diet mediates hepatic insulin resistance via endoplasmic reticulum stress
Chunjie Jiang, Shanshan Zhang, Dan Li, Li Chen, Ying Zhao, Guibin Mei, Jingjing Liu, Yuhan Tang, Chao Gao, Ping Yao
Food and Chemical Toxicology.2020; 140: 111329. CrossRef - Dipeptidyl peptidase-4 inhibitor protects against non-alcoholic steatohepatitis in mice by targeting TRAIL receptor-mediated lipoapoptosis via modulating hepatic dipeptidyl peptidase-4 expression
Minyoung Lee, Eugene Shin, Jaehyun Bae, Yongin Cho, Ji-Yeon Lee, Yong-ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha
Scientific Reports.2020;[Epub] CrossRef - Use of fenofibrate on cardiovascular outcomes in statin users with metabolic syndrome: propensity matched cohort study
Nam Hoon Kim, Ki Hoon Han, Jimi Choi, Juneyoung Lee, Sin Gon Kim
BMJ.2019; : l5125. CrossRef - Inhibition of the Low Molecular Weight Protein Tyrosine Phosphatase (LMPTP) as a Potential Therapeutic Strategy for Hepatic Progenitor Cells Lipotoxicity—Short Communication
Michalina Alicka, Katarzyna Kornicka-Garbowska, Michael Roecken, Krzysztof Marycz
International Journal of Molecular Sciences.2019; 20(23): 5873. CrossRef - Myricetin prevents thapsigargin-induced CDK5-P66Shc signalosome mediated pancreatic β-cell dysfunction
Udayakumar Karunakaran, Ji Eun Lee, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Free Radical Biology and Medicine.2019; 141: 59. CrossRef - Spontaneous ketonuria and risk of incident diabetes: a 12 year prospective study
Gyuri Kim, Sang-Guk Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Ele Ferrannini, Yong-ho Lee, Nam H. Cho
Diabetologia.2019; 62(5): 779. CrossRef - CCAAT/enhancer binding protein homologous protein knockdown alleviates hypoxia-induced myocardial injury in rat cardiomyocytes exposed to high glucose
Wenqi Yang, Fang Wu, Ting Luo, Yuelan Zhang
Experimental and Therapeutic Medicine.2018;[Epub] CrossRef - Association of changes in ER stress-mediated signaling pathway with lead-induced insulin resistance and apoptosis in rats and their prevention by A-type dimeric epigallocatechin-3-gallate
Chan-Min Liu, Jie-Qiong Ma, Jian-Mei Sun, Zhao-Jun Feng, Chao Cheng, Wei Yang, Hong Jiang
Food and Chemical Toxicology.2017; 110: 325. CrossRef
- Duality of Nrf2 in iron-overload cardiomyopathy
- Genetics
APO A2 -265T /C Polymorphism Is Associated with Increased Inflammatory Responses in Patients with Type 2 Diabetes Mellitus- Fariba Koohdani, Haleh Sadrzadeh-Yeganeh, Mahmoud Djalali, Mohammadreza Eshraghian, Elham Zamani, Gity Sotoudeh, Mohammad-Ali Mansournia, Laleh Keramat
- Diabetes Metab J. 2016;40(3):222-229. Published online June 20, 2016
- DOI: https://doi.org/10.4093/dmj.2016.40.3.222
- 3,141 View
- 28 Download
- 12 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Apolipoprotein A2 (
APO A2 ) is the second most abundant structural apolipoprotein in high density lipoprotein. Several studies have examined the possible effect ofAPO A2 on atherosclerosis incidence. Due to the role of inflammation in atherosclerosis, we aimed to determine the relationship betweenAPO A2 -265T/C polymorphism and inflammation as a risk factor in type 2 diabetes mellitus (T2DM) patients.Methods In total, 180 T2DM patients, with known
APO A2 -265T/C polymorphism, were recruited for this comparative study and were grouped equally based on their genotypes. Dietary intakes, anthropometric parameters, lipid profile, and inflammatory markers (i.e., pentraxin 3 [PTX3], high-sensitivity C-reactive protein [hs-CRP], and interleukin 18) were measured. The data were analyzed using an independentt -test, a chi-square test, and the analysis of covariance.Results After adjusting for confounding factors, in the entire study population and in the patients with or without obesity, the patients with the CC genotype showed higher hs-CRP (
P =0.001,P =0.008, andP =0.01, respectively) and lower PTX3 (P =0.01,P =0.03, andP =0.04, respectively) in comparison with the T allele carriers. In the patients with the CC genotype, no significant differences were observed in the inflammatory markers between the obese or non-obese patients. However, regarding the T allele carriers, the plasma hs-CRP level was significantly higher in the obese patients compared to the non-obese patients (P =0.01).Conclusion In the T2DM patients, the CC genotype could be considered as a risk factor and the T allele as a protective agent against inflammation, which the latter effect might be impaired by obesity. Our results confirmed the anti-atherogenic effect of
APO A2 , though more studies are required to establish this effect.-
Citations
Citations to this article as recorded by- Proteomic Profiling of Extracellular Vesicles Isolated from Plasma and Peritoneal Exudate in Mice Induced by Crotalus scutulatus scutulatus Crude Venom and Its Purified Cysteine-Rich Secretory Protein (Css-CRiSP)
Armando Reyes, Joseph D. Hatcher, Emelyn Salazar, Jacob Galan, Anton Iliuk, Elda E. Sanchez, Montamas Suntravat
Toxins.2023; 15(7): 434. CrossRef - ApoA2–256T > C polymorphism interacts with Healthy Eating Index, Dietary Quality Index-International and Dietary Phytochemical Index to affect biochemical markers among type 2 diabetic patients
Zahra Esmaeily, Gity Sotoudeh, Masoumeh Rafiee, Fariba Koohdani
British Journal of Nutrition.2022; 127(9): 1343. CrossRef - Interaction between Apo A-II –265T > C polymorphism and dietary total antioxidant capacity on some oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus
Banafsheh Jafari Azad, Mehdi Yaseri, Elnaz Daneshzad, Fariba Koohdani
British Journal of Nutrition.2022; 128(1): 13. CrossRef - Deletion allele of Apo B gene is associated with higher inflammation, oxidative stress and dyslipidemia in obese type 2 diabetic patients: an analytical cross-sectional study
Nasim Mokhtary, Seyedeh Neda Mousavi, Gity Sotoudeh, Mostafa Qorbani, Maryam Dehghani, Fariba Koohdani
BMC Endocrine Disorders.2022;[Epub] CrossRef - Interaction between Apo A-II -265T>C polymorphism and dietary total antioxidant capacity on some anthropometric indices and serum lipid profile in patients with type 2 diabetes mellitus
Banafsheh Jafari Azad, Mehdi Yaseri, Elnaz Daneshzad, Fariba Koohdani
Journal of Nutritional Science.2021;[Epub] CrossRef - A personalised diet study: The interaction between ApoA2 −265T > C polymorphism and dietary inflammatory index on oxidative and inflammatory markers and lipid profile in patients with type 2 diabetes mellitus: A cross‐sectional study
Elmira Karimi, Pourya Tondkar, Gity Sotoudeh, Mostafa Qorbani, Masoumeh Rafiee, Fariba Koohdani
International Journal of Clinical Practice.2021;[Epub] CrossRef - Interaction between the dietary indices and PPAR‐γ Pro12Ala gene variants on cardiovascular risk factors in patients with type 2 diabetes mellitus
Faezeh Abaj, Gity Sotoudeh, Elmira Karimi, Masoumeh Rafiee, Fariba Koohdani
International Journal of Clinical Practice.2021;[Epub] CrossRef - A Genetic Score of Predisposition to Low-Grade Inflammation Associated with Obesity May Contribute to Discern Population at Risk for Metabolic Syndrome
Sebastià Galmés, Margalida Cifre, Andreu Palou, Paula Oliver, Francisca Serra
Nutrients.2019; 11(2): 298. CrossRef - Study of the relationship between APOA-II −265T>C polymorphism and HDL function in response to weight loss in overweight and obese type 2 diabetic patients
Masoumeh Moradi, Maryam Mahmoudi, Ahmad Saedisomeolia, Mohammad Ali Mansournia, Roxana Zahirihashemi, Fariba Koohdani
Clinical Nutrition.2018; 37(3): 965. CrossRef - Apolipoprotein A-II induces acute-phase response associated AA amyloidosis in mice through conformational changes of plasma lipoprotein structure
Mu Yang, Yingye Liu, Jian Dai, Lin Li, Xin Ding, Zhe Xu, Masayuki Mori, Hiroki Miyahara, Jinko Sawashita, Keiichi Higuchi
Scientific Reports.2018;[Epub] CrossRef - Prenatal Exposure to Lipopolysaccharide Induces PTX3 Expression and Results in Obesity in Mouse Offspring
Shugang Qin, Xin Chen, Meng Gao, Jianzhi Zhou, Xiaohui Li
Inflammation.2017; 40(6): 1847. CrossRef
- Proteomic Profiling of Extracellular Vesicles Isolated from Plasma and Peritoneal Exudate in Mice Induced by Crotalus scutulatus scutulatus Crude Venom and Its Purified Cysteine-Rich Secretory Protein (Css-CRiSP)
- Clinical Care/Education
- Clinical Characteristics and Metabolic Predictors of Rapid Responders to Dipeptidyl Peptidase-4 Inhibitor as an Add-on Therapy to Sulfonylurea and Metformin
- Ye An Kim, Won Sang Yoo, Eun Shil Hong, Eu Jeong Ku, Kyeong Seon Park, Soo Lim, Young Min Cho, Kyong Soo Park, Hak Chul Jang, Sung Hee Choi
- Diabetes Metab J. 2015;39(6):489-497. Published online November 27, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.6.489
- 3,523 View
- 39 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Dipeptidyl peptidase-4 (DPP-4) inhibitor add-on therapy is a new option for patients with inadequately controlled type 2 diabetes who are taking combined metformin and sulfonylurea (SU). We evaluated the efficacy and safety of this triple therapy and the characteristics of rapid responders and hypoglycemia-prone patients.
Methods We included 807 patients with type 2 diabetes who were prescribed a newly added DPP-4 inhibitor to ongoing metformin and SU in 2009 to 2011. Glycemia and other metabolic parameters at baseline, 12, 24, and 52 weeks, as well as episodes of hypoglycemia were analyzed. Rapid responders were defined as patients with ≥25% reduction in glycosylated hemoglobin (HbA1c) within 12 weeks.
Results At baseline, while on the submaximal metformin and SU combination, the mean HbA1c level was 8.4%. Twelve weeks after initiation of DPP-4 inhibitor add-on, 269 patients (34.4%) achieved an HbA1c level ≤7%. Sixty-six patients (8.2%, 47 men) were rapid responders. The duration of diabetes was shorter in rapid responders, and their baseline fasting plasma glucose (FPG), HbA1c, C-peptide, and homeostasis model assessment of insulin resistance were significantly higher. Patients who experienced hypoglycemia after taking DPP-4 inhibitor add-on were more likely to be female, to have a lower body weight and lower triglyceride and FPG levels, and to have higher homeostasis model assessment of β-cells.
Conclusion An oral hypoglycemic triple agent combination including a DPP-4 inhibitor was effective in patients with uncontrolled diabetes. Proactive dose reduction of SU should be considered when a DPP-4 inhibitor is added for rapid responders and hypoglycemia-prone patients.
-
Citations
Citations to this article as recorded by- A genetic variant in GLP1R is associated with response to DPP-4 inhibitors in patients with type 2 diabetes
Eugene Han, Hye Sun Park, Obin Kwon, Eun Yeong Choe, Hye Jin Wang, Yong-ho Lee, Sang-Hak Lee, Chul Hoon Kim, Lee-Kyung Kim, Soo Heon Kwak, Kyong Soo Park, Chul Sik Kim, Eun Seok Kang
Medicine.2016; 95(44): e5155. CrossRef
- A genetic variant in GLP1R is associated with response to DPP-4 inhibitors in patients with type 2 diabetes
- The Role of Heat Shock Response in Insulin Resistance and Diabetes
- Tatsuya Kondo, Hiroyuki Motoshima, Motoyuki Igata, Junji Kawashima, Takeshi Matsumura, Hirofumi Kai, Eiichi Araki
- Diabetes Metab J. 2014;38(2):100-106. Published online April 18, 2014
- DOI: https://doi.org/10.4093/dmj.2014.38.2.100
- 4,640 View
- 32 Download
- 7 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The expansion of life-style related diseases, such as metabolic syndrome (MS) and type 2 diabetes mellitus (T2DM), appears to be unstoppable. It is also difficult to cease their complications in spite of many antidiabetic medications or intervention of public administration. We and our collaborators found that physical medicine using simultaneous stimulation of heat with mild electric current activates heat shock response, thereby reducing visceral adiposity, insulin resistance, chronic inflammation and improving glucose homeostasis in mice models of T2DM, as well as in humans with MS or T2DM. This combination therapy exerts novel action on insulin signaling, β-cell protection and body compositions, and may provide a new therapeutic alternative in diabetic treatment strategy.
-
Citations
Citations to this article as recorded by- Proteostasis defects: Medicinal challenges of imperfect aging & neurodegeneration
Prashant Kumar, Akash Choudhary, Sumit Kinger, Yuvraj Anandrao Jagtap, Ankur Rakesh Dubey, Ravi Kumar Gutti, Deepak Chitkara, Anil K. Suresh, Amit Mishra
Translational Medicine of Aging.2023; 7: 87. CrossRef - New opportunities of therapeutic hyperthermia (literature review)
Orazakhmet K. Kurpeshev
Russian Journal of Physiotherapy, Balneology and Rehabilitation.2022; 20(5): 429. CrossRef - eIF3j inhibits translation of a subset of circular RNAs in eukaryotic cells
Zhenxing Song, Jiamei Lin, Rui Su, Yu Ji, Ruirui Jia, Shi Li, Ge Shan, Chuan Huang
Nucleic Acids Research.2022; 50(20): 11529. CrossRef - Are Heat Shock Proteins an Important Link between Type 2 Diabetes and Alzheimer Disease?
Joanne Elizabeth Rowles, Kevin Noel Keane, Thiago Gomes Heck, Vinicius Cruzat, Giuseppe Verdile, Philip Newsholme
International Journal of Molecular Sciences.2020; 21(21): 8204. CrossRef - The effect of passive heating on heat shock protein 70 and interleukin-6: A possible treatment tool for metabolic diseases?
S. H. Faulkner, S. Jackson, G. Fatania, C. A. Leicht
Temperature.2017; 4(3): 292. CrossRef - Emerging Role of Nitric Oxide and Heat Shock Proteins in Insulin Resistance
Marisa Nile Molina, León Ferder, Walter Manucha
Current Hypertension Reports.2016;[Epub] CrossRef - αB-crystallin and HspB2 deficiency is protective from diet-induced glucose intolerance
Daniel J. Toft, Miles Fuller, Matthew Schipma, Feng Chen, Vincent L. Cryns, Brian T. Layden
Genomics Data.2016; 9: 10. CrossRef - DNAJB3/HSP-40 cochaperone improves insulin signaling and enhances glucose uptake in vitro through JNK repression
Mohamed Abu-Farha, Preethi Cherian, Irina Al-Khairi, Ali Tiss, Abdelkrim Khadir, Sina Kavalakatt, Samia Warsame, Mohammed Dehbi, Kazem Behbehani, Jehad Abubaker
Scientific Reports.2015;[Epub] CrossRef - Reduced nuclear protein 1 expression improves insulin sensitivity and protects against diet-induced glucose intolerance through up-regulation of heat shock protein 70
H.C. Barbosa-Sampaio, R. Drynda, B. Liu, A.M. Rodriguez De Ledesma, C. Malicet, J.L. Iovanna, P.M. Jones, D.S. Muller, S.J. Persaud
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2015; 1852(5): 962. CrossRef - Mild Electrical Stimulation Increases Stress Resistance and Suppresses Fat Accumulation via Activation of LKB1-AMPK Signaling Pathway in C. elegans
Shingo Matsuyama, Masataka Moriuchi, Mary Ann Suico, Shuichiro Yano, Saori Morino-Koga, Tsuyoshi Shuto, Kunitoshi Yamanaka, Tatsuya Kondo, Eiichi Araki, Hirofumi Kai, Deyu Fang
PLoS ONE.2014; 9(12): e114690. CrossRef
- Proteostasis defects: Medicinal challenges of imperfect aging & neurodegeneration

 KDA
KDA
 First
First Prev
Prev





