
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Metabolic Risk/Epidemiology
- A Composite Blood Biomarker Including AKR1B10 and Cytokeratin 18 for Progressive Types of Nonalcoholic Fatty Liver Disease
- Seung Joon Choi, Sungjin Yoon, Kyoung-Kon Kim, Doojin Kim, Hye Eun Lee, Kwang Gi Kim, Seung Kak Shin, Ie Byung Park, Seong Min Kim, Dae Ho Lee
- Received June 18, 2023 Accepted August 16, 2023 Published online February 1, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0189 [Epub ahead of print]

- 780 View
- 50 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We aimed to evaluate whether composite blood biomarkers including aldo-keto reductase family 1 member B10 (AKR1B10) and cytokeratin 18 (CK-18; a nonalcoholic steatohepatitis [NASH] marker) have clinically applicable performance for the diagnosis of NASH, advanced liver fibrosis, and high-risk NASH (NASH+significant fibrosis).
Methods
A total of 116 subjects including healthy control subjects and patients with biopsy-proven nonalcoholic fatty liver disease (NAFLD) were analyzed to assess composite blood-based and imaging-based biomarkers either singly or in combination.
Results
A composite blood biomarker comprised of AKR1B10, CK-18, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) showed excellent performance for the diagnosis of, NASH, advanced fibrosis, and high-risk NASH, with area under the receiver operating characteristic curve values of 0.934 (95% confidence interval [CI], 0.888 to 0.981), 0.902 (95% CI, 0.832 to 0.971), and 0.918 (95% CI, 0.862 to 0.974), respectively. However, the performance of this blood composite biomarker was inferior to that various magnetic resonance (MR)-based composite biomarkers, such as proton density fat fraction/MR elastography- liver stiffness measurement (MRE-LSM)/ALT/AST for NASH, MRE-LSM+fibrosis-4 index for advanced fibrosis, and the known MR imaging-AST (MAST) score for high-risk NASH.
Conclusion
Our blood composite biomarker can be useful to distinguish progressive forms of NAFLD as an initial noninvasive test when MR-based tools are not available.
- Metabolic Risk/Epidemiology
-
- Magnetic Resonance-Based Assessments Better Capture Pathophysiologic Profiles and Progression in Nonalcoholic Fatty Liver Disease
- Seung Joon Choi, Seong Min Kim, Yun Soo Kim, Oh Sang Kwon, Seung Kak Shin, Kyoung Kon Kim, Kiyoung Lee, Ie Byung Park, Cheol Soo Choi, Dong Hae Chung, Jaehun Jung, MunYoung Paek, Dae Ho Lee
- Diabetes Metab J. 2021;45(5):739-752. Published online October 28, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0137
- 8,735 View
- 219 Download
- 13 Web of Science
- 15 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
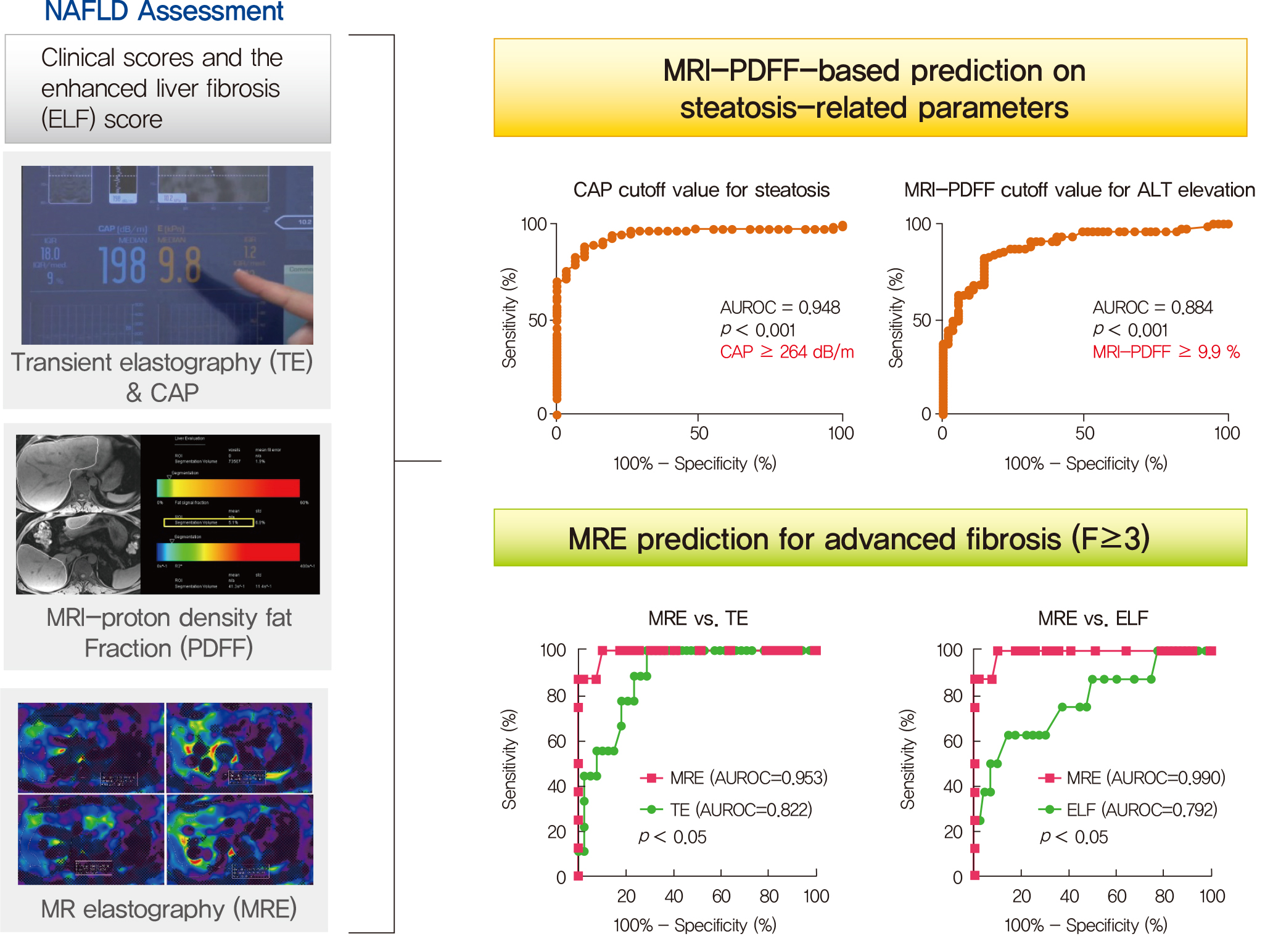
Several noninvasive tools are available for the assessment of nonalcoholic fatty liver disease (NAFLD) including clinical and blood biomarkers, transient elastography (TE), and magnetic resonance imaging (MRI) techniques, such as proton density fat fraction (MRI-PDFF) and magnetic resonance elastography (MRE). In the present study, we aimed to evaluate whether magnetic resonance (MR)-based examinations better discriminate the pathophysiologic features and fibrosis progression in NAFLD than other noninvasive methods.
Methods
A total of 133 subjects (31 healthy volunteers and 102 patients with NAFLD) were subjected to clinical and noninvasive NAFLD evaluation, with additional liver biopsy in some patients (n=54).
Results
MRI-PDFF correlated far better with hepatic fat measured by MR spectroscopy (r=0.978, P<0.001) than with the TE controlled attenuation parameter (CAP) (r=0.727, P<0.001). In addition, MRI-PDFF showed stronger correlations with various pathophysiologic parameters for cellular injury, glucose and lipid metabolism, and inflammation, than the TE-CAP. The MRI-PDFF and TE-CAP cutoff levels associated with abnormal elevation of serum alanine aminotransferase were 9.9% and 270 dB/m, respectively. The MRE liver stiffness measurement (LSM) showed stronger correlations with liver enzymes, platelets, complement component 3, several clinical fibrosis scores, and the enhanced liver fibrosis (ELF) score than the TE-LSM. In an analysis of only biopsied patients, MRE performed better in discriminating advanced fibrosis with a cutoff value of 3.9 kPa than the TE (cutoff 8.1 kPa) and ELF test (cutoff 9.2 kPa).
Conclusion
Our results suggest that MRI-based assessment of NAFLD is the best non-invasive tool that captures the histologic, pathophysiologic and metabolic features of the disease. -
Citations
Citations to this article as recorded by- A Novel Score Based on Controlled Attenuation Parameter Accurately Predicts Hepatic Steatosis in Individuals With Metabolic Dysfunction Associated Steatotic Liver Disease: A Derivation and Independent Validation Study
Zi-Ming An, Qiao-Hong Liu, Xin-Jian Ye, Qian Zhang, Hua-Fu Pei, Xin Xin, Jie Yuan, Qian Huang, Kun Liu, Fang Lu, Zhi-Han Yan, Yu Zhao, Yi-Yang Hu, Ming-Hua Zheng, Qin Feng
Clinical and Translational Gastroenterology.2024; 15(3): e00680. CrossRef - Imaging Methods Applicable in the Diagnostics of Alzheimer’s Disease, Considering the Involvement of Insulin Resistance
Petra Hnilicova, Ema Kantorova, Stanislav Sutovsky, Milan Grofik, Kamil Zelenak, Egon Kurca, Norbert Zilka, Petra Parvanovova, Martin Kolisek
International Journal of Molecular Sciences.2023; 24(4): 3325. CrossRef - Polyunsaturated and Saturated Oxylipin Plasma Levels Allow Monitoring the Non-Alcoholic Fatty Liver Disease Progression to Severe Stages
Miguel D. Ferrer, Clara Reynés, Margalida Monserrat-Mesquida, Magdalena Quetglas-Llabrés, Cristina Bouzas, Silvia García, David Mateos, Miguel Casares, Cristina Gómez, Lucía Ugarriza, Josep A. Tur, Antoni Sureda, Antoni Pons
Antioxidants.2023; 12(3): 711. CrossRef - An individual patient data meta-analysis to determine cut-offs for and confounders of NAFLD-fibrosis staging with magnetic resonance elastography
Jia-xu Liang, Javier Ampuero, Hao Niu, Kento Imajo, Mazen Noureddin, Jaideep Behari, Dae Ho Lee, Richard L. Ehman, Fredrik Rorsman, Johan Vessby, Juan R. Lacalle, Ferenc E. Mózes, Michael Pavlides, Quentin M. Anstee, Stephen A. Harrison, Javier Castell, R
Journal of Hepatology.2023; 79(3): 592. CrossRef - Relationship between controlled attenuated parameter and magnetic resonance imaging–proton density fat fraction for evaluating hepatic steatosis in patients with NAFLD
Ziming An, Qiaohong Liu, Wenli Zeng, Yan Wang, Qian Zhang, Huafu Pei, Xin Xin, Shuohui Yang, Fang Lu, Yu Zhao, Yiyang Hu, Qin Feng
Hepatology Communications.2022; 6(8): 1975. CrossRef - Noninvasive imaging of hepatic dysfunction: A state-of-the-art review
Ting Duan, Han-Yu Jiang, Wen-Wu Ling, Bin Song
World Journal of Gastroenterology.2022; 28(16): 1625. CrossRef - Diagnosis and Pathogenesis of Sarcopenia in Chronic Liver Disease Using Liver Magnetic Resonance Imaging
Atsushi Nakamura, Tsubasa Yoshimura, Tomomi Sato, Takeshi Ichikawa
Cureus.2022;[Epub] CrossRef - Plasma Aldo-Keto Reductase Family 1 Member B10 as a Biomarker Performs Well in the Diagnosis of Nonalcoholic Steatohepatitis and Fibrosis
Aron Park, Seung Joon Choi, Sungjin Park, Seong Min Kim, Hye Eun Lee, Minjae Joo, Kyoung Kon Kim, Doojin Kim, Dong Hae Chung, Jae Been Im, Jaehun Jung, Seung Kak Shin, Byung-Chul Oh, Cheolsoo Choi, Seungyoon Nam, Dae Ho Lee
International Journal of Molecular Sciences.2022; 23(9): 5035. CrossRef - Contribution of a genetic risk score to ethnic differences in fatty liver disease
Maddie J. Kubiliun, Jonathan C. Cohen, Helen H. Hobbs, Julia Kozlitina
Liver International.2022; 42(10): 2227. CrossRef - Plasma Metabolomics and Machine Learning-Driven Novel Diagnostic Signature for Non-Alcoholic Steatohepatitis
Moongi Ji, Yunju Jo, Seung Joon Choi, Seong Min Kim, Kyoung Kon Kim, Byung-Chul Oh, Dongryeol Ryu, Man-Jeong Paik, Dae Ho Lee
Biomedicines.2022; 10(7): 1669. CrossRef - Updated S2k Clinical Practice Guideline on Non-alcoholic Fatty Liver Disease (NAFLD) issued by the German Society of Gastroenterology, Digestive and Metabolic Diseases (DGVS) – April 2022 – AWMF Registration No.: 021–025
Zeitschrift für Gastroenterologie.2022; 60(09): e733. CrossRef - Aktualisierte S2k-Leitlinie nicht-alkoholische Fettlebererkrankung der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS) – April 2022 – AWMF-Registernummer: 021–025
E. Roeb, A. Canbay, H. Bantel, J. Bojunga, J. de Laffolie, M. Demir, U. W. Denzer, A. Geier, W. P. Hofmann, C. Hudert, T. Karlas, M. Krawczyk, T. Longerich, T. Luedde, M. Roden, J. Schattenberg, M. Sterneck, A. Tannapfel, P. Lorenz, F. Tacke
Zeitschrift für Gastroenterologie.2022; 60(09): 1346. CrossRef - Ultrasound Methods for the Assessment of Liver Steatosis: A Critical Appraisal
Dorotea Bozic, Kristian Podrug, Ivana Mikolasevic, Ivica Grgurevic
Diagnostics.2022; 12(10): 2287. CrossRef - Significance of liver fat loss in chronic liver disease: Usefulness of hepatic proton density fat fraction measurement by magnetic resonance imaging in evaluating malnutrition
Atsushi Nakamura, Haruka Okada, Tsubasa Yoshimura, Manami Deguchi, Yuei Hosokawa, Tomomi Satoh, Takeshi Ichikawa, Keiji Okuyama, Yoshihiro Yoshioka, Hitoshi Asakura
Kanzo.2021; 62(9): 525. CrossRef - Screening for nonalcoholic fatty liver disease-when, who and how?
Christoph G Dietrich, Monika Rau, Andreas Geier
World Journal of Gastroenterology.2021; 27(35): 5803. CrossRef
- A Novel Score Based on Controlled Attenuation Parameter Accurately Predicts Hepatic Steatosis in Individuals With Metabolic Dysfunction Associated Steatotic Liver Disease: A Derivation and Independent Validation Study
- Drug/Regimen
-
- Glucagon-Like Peptide-1 Receptor Agonist Differentially Affects Brain Activation in Response to Visual Food Cues in Lean and Obese Individuals with Type 2 Diabetes Mellitus
- Jae Hyun Bae, Hyung Jin Choi, Kang Ik Kevin Cho, Lee Kyung Kim, Jun Soo Kwon, Young Min Cho
- Diabetes Metab J. 2020;44(2):248-259. Published online November 4, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0018
- 7,399 View
- 222 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background To investigate the effects of a glucagon-like peptide-1 receptor agonist on functional brain activation in lean and obese individuals with type 2 diabetes mellitus (T2DM) in response to visual food cues.
Methods In a randomized, single-blinded, crossover study, 15 lean and 14 obese individuals with T2DM were administered lixisenatide or normal saline subcutaneously with a 1-week washout period. We evaluated brain activation in response to pictures of high-calorie food, low-calorie food, and nonfood using functional magnetic resonance imaging and measured appetite and caloric intake in participants who were given access to an
ad libitum buffet.Results Obese individuals with T2DM showed significantly greater activation of the hypothalamus, pineal gland, parietal cortex (high-calorie food vs. low-calorie food,
P <0.05), orbitofrontal cortex (high-calorie food vs. nonfood,P <0.05), and visual cortex (food vs. nonfood,P <0.05) than lean individuals with T2DM. Lixisenatide injection significantly reduced the functional activation of the fusiform gyrus and lateral ventricle in obese individuals with T2DM compared with that in lean individuals with T2DM (nonfood vs. high-calorie food,P <0.05). In addition, in individuals who decreased their caloric intake after lixisenatide injection, there were significant interaction effects between group and treatment in the posterior cingulate, medial frontal cortex (high-calorie food vs. low-calorie food,P <0.05), hypothalamus, orbitofrontal cortex, and temporal lobe (food vs. nonfood,P <0.05).Conclusion Brain responses to visual food cues were different in lean and obese individuals with T2DM. In addition, acute administration of lixisenatide differentially affected functional brain activation in these individuals, especially in those who decreased their caloric intake after lixisenatide injection.
-
Citations
Citations to this article as recorded by- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Jae Hyun Bae
Diabetes & Metabolism Journal.2024; 48(1): 157. CrossRef - Diabetes remission and relapse following an intensive metabolic intervention combining insulin glargine/lixisenatide, metformin and lifestyle approaches: Results of a randomised controlled trial
Natalia McInnes, Stephanie Hall, Heather A. Lochnan, Stewart B. Harris, Zubin Punthakee, Ronald J. Sigal, Irene Hramiak, Mohammed Azharuddin, Joanne F. Liutkus, Jean‐François Yale, Farah Sultan, Ada Smith, Rose E. Otto, Diana Sherifali, Yan Yun Liu, Hertz
Diabetes, Obesity and Metabolism.2023; 25(11): 3347. CrossRef - Glucagon-like peptide-1 analog therapy in rare genetic diseases: monogenic obesity, monogenic diabetes, and spinal muscular atrophy
Hussein Zaitoon, Ronit Lubetzky, Achiya Z. Amir, Hadar Moran-Lev, Liora Sagi, Michal Yacobi-Bach, Ophir Borger, Efrat Chorna, Yael Lebenthal, Avivit Brener
Acta Diabetologica.2023; 60(8): 1099. CrossRef - What can functional brain imaging teach us about remission of type 2 diabetes?
Dhruti Hirani, Shahd Alabdulkader, Alexander. D. Miras, Victoria Salem
Diabetic Medicine.2023;[Epub] CrossRef - Fasting oxyntomodulin, glicentin, and gastric inhibitory polypeptide levels are associated with activation of reward‐ and attention‐related brain centres in response to visual food cues in adults with obesity: A cross‐sectional functional MRI study
Nikolaos Perakakis, Olivia M. Farr, Christos S. Mantzoros
Diabetes, Obesity and Metabolism.2021; 23(5): 1202. CrossRef - Aberrant Brain Functional Connectivity Strength and Effective Connectivity in Patients with Type 2 Diabetes Mellitus
Xi Guo, Su Wang, Yu-Chen Chen, Heng-Le Wei, Gang-Ping Zhou, Yu-Sheng Yu, Xindao Yin, Kun Wang, Hong Zhang, Eusebio Chiefari
Journal of Diabetes Research.2021; 2021: 1. CrossRef
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)

 KDA
KDA
 First
First Prev
Prev





