
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Review
- Complications
- Pharmacological and Nonpharmacological Treatments for Painful Diabetic Peripheral Neuropathy
- Han Na Jang, Tae Jung Oh
- Diabetes Metab J. 2023;47(6):743-756. Published online September 6, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0018
- 4,390 View
- 576 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
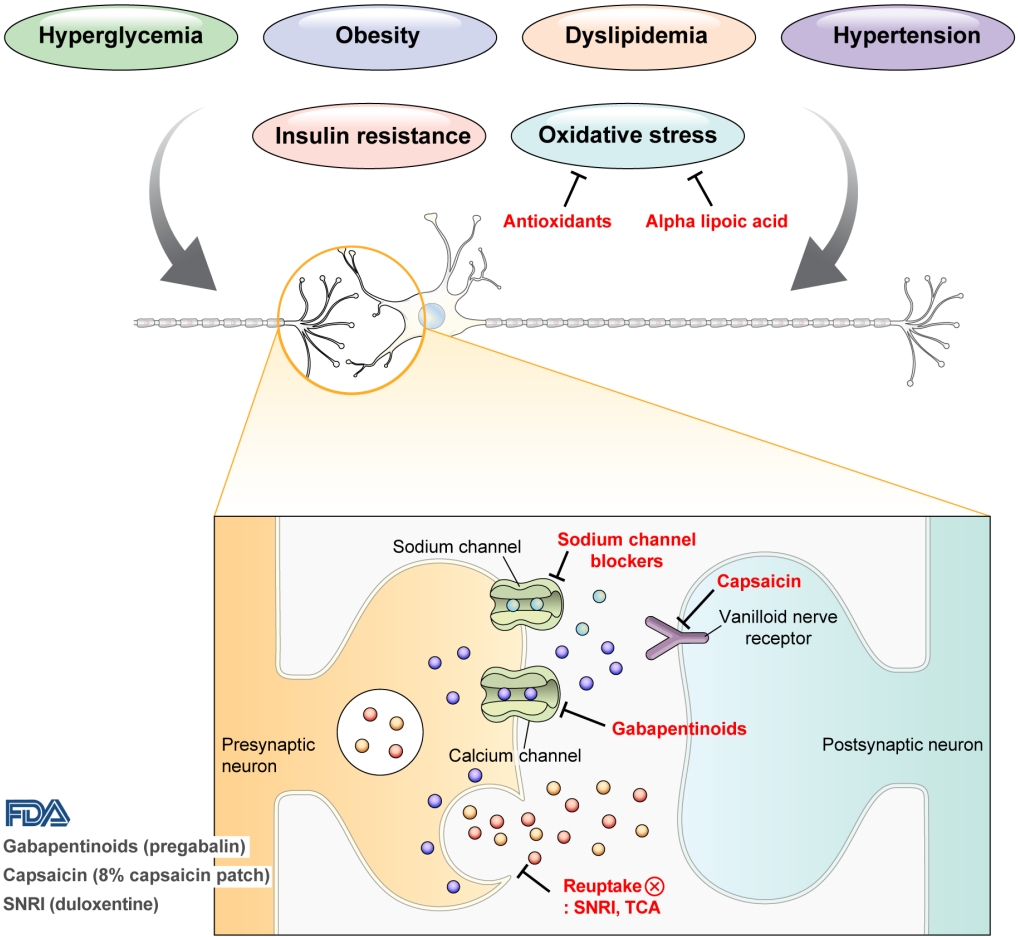
ePub - Diabetic peripheral neuropathy (DPN) is one of the most prevalent chronic complications of diabetes. The lifetime prevalence of DPN is thought to be >50%, and 15%–25% of patients with diabetes experience neuropathic pain, referred to as “painful DPN.” Appropriate treatment of painful DPN is important because this pain contributes to a poor quality of life by causing sleep disturbance, anxiety, and depression. The basic principle for the management of painful DPN is to control hyperglycemia and other modifiable risk factors, but these may be insufficient for preventing or improving DPN. Because there is no promising diseasemodifying medication for DPN, the pain itself needs to be managed when treating painful DPN. Drugs for neuropathic pain, such as gabapentinoids, serotonin–norepinephrine reuptake inhibitors, tricyclic antidepressants, alpha-lipoic acid, sodium channel blockers, and topical capsaicin, are used for the management of painful DPN. The U.S. Food and Drug Administration (FDA) has approved pregabalin, duloxetine, tapentadol, and the 8% capsaicin patch as drugs for the treatment of painful DPN. Recently, spinal cord stimulation using electrical stimulation is approved by the FDA for the treatment for painful DPN. This review describes the currently available pharmacological and nonpharmacological treatments for painful DPN.
-
Citations
Citations to this article as recorded by- J-2156, a small molecule somatostatin type 4 receptor agonist, alleviated hindpaw hypersensitivity in the streptozotocin-induced rat model of painful diabetic neuropathy but with a 2-fold decrease in potency at an advanced stage in the model, mimicking mo
A. Kuo, M. Z. Imam, R. Li, L. Lin, A. Raboczyj, A. E. Bohmer, J. R. Nicholson, L. Corradini, M. T. Smith
Frontiers in Pharmacology.2024;[Epub] CrossRef - The Chronic Wound–Related Pain Model
Kevin Woo
Clinics in Geriatric Medicine.2024;[Epub] CrossRef
- J-2156, a small molecule somatostatin type 4 receptor agonist, alleviated hindpaw hypersensitivity in the streptozotocin-induced rat model of painful diabetic neuropathy but with a 2-fold decrease in potency at an advanced stage in the model, mimicking mo
Original Article
- Analgesic Effects of DA-5018, a New Capsaicin Derivative, in Hyperalgesia of Experimental Diabetic Neuropathy.
- Eun Ju Bae, Soon How Kim, Moon Ho Son, Hee Kee Kim, Myeong Soo Shin, Hyun Ji Kim, Won Bae Kim
- Korean Diabetes J. 1997;21(1):91-101. Published online January 1, 2001
- 1,060 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Painful peripheral neuropathy is one of the most common complications of diabetes and not responsive to conventional analgesics. Capsaicin cream has been used to treat the pain associated with diabetic neuropathy, rheumatoid arthritis, osteoarthritis and postherpetic neuralgia. But its common side effect, burning sensation, limits the use of it. DA-5018 is a newly synthesized capsaicin derivative which shows more potent systemic and topical analgesia than capsaicin in various animal models of acute and chronic pain, but has little skin irritaion. This study was designed to evaluate the effect of DA-5018 administered systemically or topically on hyperalgesia in streptozotocin-induced diabetic and galactosaemic rats. METHODS: One group of SD rats was treated with streptozotocin(60mg/kg, I.v.) and the pain thresholds were determined weekly by Randall-Selitto paw pressure test. And the other group of SD rats was maintained on 50%-galactose diet until 4~5 weeks and the pain thresholds were determined as well, Drugs were administered subcutaneously once a day for 7 days or topically to the paw for 5 hours a day for 10 days at a time when the hyperalgesia was already present. The increase of pain thresholds by drug was regarded as an indication of analgesia. RESULTS: Streptozotocin-diabetic rats displayed a reduction of pain threshold. Similarly, galactosefeeding resulted in significant reduction of pain threshold. It is concluded that hyperalgesia is a constant feature of sensory dysfunction in experimental models of diabetic and nutritional neuropathy. DA-5018(0.2, 0.5mg/kg, s.c.) produced significant antinociception with efficacy similar to that of capsaicin(10mg/kg, s.c.) in streptozotocin-induced hyperalgesia and furthermore, no tolerance developed for 7 days. And this analgesic effect was superior to desipramine(10mg/kg, s.c.). But ketoprofen(10mg/kg, s.c.) produced no analgesia. Topically, 0.3% DA-5018 cream was as effective as Zostrix-HP(capsaicin 0.075%) both in streptozotocin-diabetic and galactosefed rats while Kenofen gel(ketoprofen 3%) was ineffective to reduce pain. CONCLUSION: These results demonstrate the potent analgesic efficacy of DA-5018 in diabetic pain models and suggest that topical DA-5018 cream may relieves pain caused by diabetic neuropathy offering an alternative for patients not responsive to other treatments or unable to tolerate capsaicin.

 KDA
KDA
 First
First Prev
Prev





