- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 38(6); 2014 > Article
-
ReviewPathophysiology A Gut Feeling to Cure Diabetes: Potential Mechanisms of Diabetes Remission after Bariatric Surgery
- Young Min Cho
-
Diabetes & Metabolism Journal 2014;38(6):406-415.
DOI: https://doi.org/10.4093/dmj.2014.38.6.406
Published online: December 15, 2014
Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- Corresponding author: Young Min Cho. Department of Internal Medicine, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 110-744, Korea. ymchomd@snu.ac.kr
Copyright © 2014 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- A cure for type 2 diabetes was once a mere dream but has now become a tangible and achievable goal with the unforeseen success of bariatric surgery in the treatment of both obesity and type 2 diabetes. Popular bariatric procedures such as Roux-en-Y gastric bypass and sleeve gastrectomy exhibit high rates of diabetes remission or marked improvement in glycemic control. However, the mechanism of diabetes remission following these procedures is still elusive and appears to be very complex and encompasses multiple anatomical and physiological changes. In this article, calorie restriction, improved β-cell function, improved insulin sensitivity, and alterations in gut physiology, bile acid metabolism, and gut microbiota are reviewed as potential mechanisms of diabetes remission after Roux-en-Y gastric bypass and sleeve gastrectomy.
- A potential cure for diabetes has arisen in an unexpected way. As diabetologists, we have tried to determine the pathophysiology of type 2 diabetes so that we can normalize glucose homeostasis without using any oral or injected medications. However, the results of our ceaseless efforts leave us far from a cure. With heart-aching disappointment in mind, we have practiced within a paradigm of "care not cure," which suggests that a cure is impossible to attain but that care is currently the best option. In 1995, Dr. Pories published a paper with a somewhat provocative title, "Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus [1]." At that time, Dr. Pories observed a drastic improvement in blood glucose levels after Roux-en-Y gastric bypass (RYGB) in obese subjects who had diabetes or impaired glucose tolerance. This was the earliest glimpse of a potential diabetes cure by surgical treatment. In a meta-analysis performed in 2004 including approximately 5,000 patients with type 2 diabetes [2], diabetes remission was observed in 76.8% of obese patients with type 2 diabetes who underwent any type of bariatric surgery. However, diabetes remission rates differed according to the type of surgery that patients received (47.9% for gastric banding, 71.6% for vertical banded gastroplasty, 83.7% for RYGB, and 98.9% for biliopancreatic diversion [BPD]) [2], which implies that the mechanism of diabetes remission is complex and encompasses a variety of anatomical, physiological, and molecular changes. In a recent randomized controlled trial with obese type 2 diabetes patients [3], the rate of diabetes remission (defined as a fasting glucose level of <100 mg/dL and an hemoglobin A1c (HbA1c) level of <6.5% with no antidiabetes medications) was 0% with medical therapy alone, 75% with RYGB, and 95% with BPD. In a 1-year randomized controlled trial in obese patients with uncontrolled type 2 diabetes [4], both RYGB and sleeve gastrectomy (SG) achieved improved glycemic control, defined as an HbA1c level of <6.0%, more frequently (42% and 37% of patients, respectively) than medical therapy alone (12% of patients). Therefore, bariatric surgery has evolved into metabolic/diabetes surgery. Furthermore, the benefits of bariatric surgery extend far beyond glycemic control. In the Swedish Obese Subjects Study, mostly composed of patients who underwent vertical banded gastroplasty, risk factors for cardiovascular diseases, including high blood pressure, dyslipidemia, hypercholesterolemia, and hyperuricemia, were greatly reduced at 2 years postsurgery, and these effects persisted over 10 years after surgery [5]. Furthermore, bariatric surgery reduced all-cause mortality [6], cardiovascular events and mortality [7], and the incidence of cancer [8]. Bariatric surgery was also effective in preventing type 2 diabetes [9] and in reducing microvascular and macrovascular complications [10]. Overall, bariatric surgery improved the quality of life in obese subjects [11]. In the following article, I would like to present a succinct review regarding the current understanding of the mechanism of diabetes remission after bariatric surgery focused on RYGB and SG.
INTRODUCTION
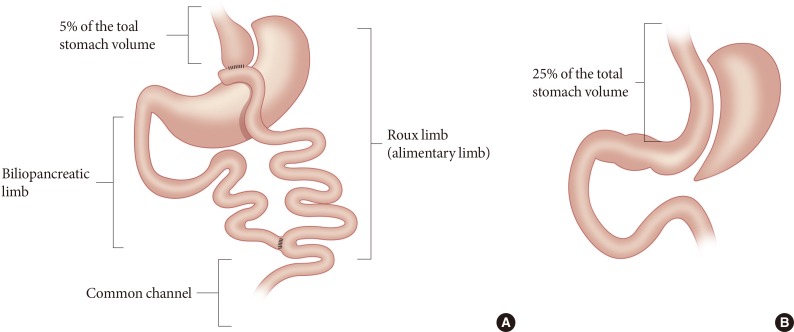
- RYGB isolates approximately 95% of the stomach from the passage of food; therefore, a gastric pouch with approximately 5% of the total stomach volume receives orally ingested food. The configuration of RYGB shown in Fig. 1A consists of the Roux limb or alimentary limb, the biliopancreatic limb, and the common channel. In the common channel, the orally ingested food meets digestive enzymes secreted by the pancreas and bile acids secreted by the hepatobiliary system. Thus, the RYGB procedure (1) restricts the amount of food intake; (2) bypasses most of the stomach, duodenum, and proximal jejunum; and (3) expedites the passage of unabsorbed nutrients to the distal intestine where glucagon-like peptide-1 (GLP-1) and peptide-YY (PYY) secreting L-cells are abundant. Indeed, GLP-1 and PYY secretion is markedly increased after RYGB.
- The SG procedure removes approximately 75% of the stomach to restrict food intake (Fig. 1B). With this surgery, the intragastric pressure increases upon ingestion of foods, which in turn increases the tension of the gastric wall [12]. Both gastric emptying and intestinal transit are markedly increased after SG [12]. Interestingly, GLP-1 secretion is noticeably augmented with SG, even though the anatomy of the gastrointestinal tract is not altered except with regard to the restriction of gastric volume. Rapid gastric emptying may contribute to the increased GLP-1 secretion. However, direct intraduodenal infusion of dextrose increased GLP-1 secretion in mice subjected to SG relative to control animals, which indicates that altered gastrointestinal physiology independent of altered gastric emptying plays an important role in the exaggerated GLP-1 response after SG [12].
CHARACTERISTICS OF RYGB AND SLEEVE GASTRECTOMY
- Decreased calorie intake and weight loss
- RYGB typically results in 35% to 40% body weight loss from baseline and 50% to 80% excess body weight loss [13]. However, a dramatic improvement in glucose control frequently occurs immediately after the surgery, usually within 1 week, when significant weight loss has not yet taken place [1,14]. Therefore, the mechanism of immediate diabetes remission or improvement appears to be weight loss-independent. However, adjustable gastric banding (AGB) is typically accompanied by a gradual improvement in glucose control in obese type 2 diabetes patients [15], which is in contrast to results observed from RYGB. The discrepancy in the time-course of diabetes remission between RYGB and AGB suggests that there are mechanisms other than weight loss per se for the rapid remission or improvement of diabetes after RYGB. However, it was shown that calorie restriction is required for rapid improvement in insulin sensitivity immediately after RYGB (within 1 week) by comparing the effects of calorie restriction and RYGB in obese subjects [16]. Similarly, in a within-subject time series study comparing the effects of calorie restriction and RYGB, both treatments resulted in similar marked improvements in glucose homeostasis in obese type 2 diabetes patients [17]. In addition, when nondiabetic obese subjects achieved 20% weight loss from baseline after either RYGB (average 16±2 weeks after surgery) or AGB (average 22±7 weeks after surgery), similar changes in β-cell function, insulin sensitivity, and gene expression in adipose tissue were observed [18], which indicates that weight loss is important in improved glucose homeostasis. However, both calorie restriction without surgical stress [16,17] and weight loss in nondiabetic subjects [18] have substantial limitations in recapitulating the processes that occur in obese type 2 diabetes patients after RYGB. Nevertheless, acute energy restriction and long-term weight loss play an important role in the improvement of glucose homeostasis following RYGB [19].
- Changes in gut physiology
- As explained earlier, RYGB causes enormous changes in the gastrointestinal anatomy, and therefore, altered gastrointestinal physiology is expected to occur after surgery. In this regard, both foregut and hindgut factors have been suggested as important players [13]. Although many factors have been demonstrated to independently contribute to the remission of diabetes from a reductionist's point of view, careful interpretation from a holistic point of view is necessary because many anatomic, physiologic, and molecular changes coalesce after bariatric surgery.
- Ghrelin is the only orexigenic gastrointestinal peptide secreted mainly from the gastric fundus. Evaluation of ghrelin secretion in patients after RYGB showed mixed results, perhaps due to different surgical techniques [13,20]. Therefore, intuitively, the role of ghrelin appears to be dispensable during diabetes remission after RYGB. However, in patients who receive SG, which removes most of the ghrelin-producing tissue, a marked and persistent decrease in ghrelin secretion is typically observed [21]. Yet, SG influences appetite, body weight, and glucose metabolism even in ghrelin knockout mice [22]. Therefore, ghrelin is unlikely to be a critical factor in diabetes remission after bariatric surgery.
- As illustrated in Fig. 1A, one of the components of RYGB is the exclusion of the duodenum and the proximal jejunum from the passage of food. To examine the role of excluding the duodenum and upper jejunum, an experimental procedure called the duodenal-jejunal bypass (DJB) surgery was created and tested in Goto-Kakizaki rats, a nonobese type 2 diabetic animal model. With this elegant surgical model, the exclusion of the duodenum and proximal jejunum without gastric volume restriction exhibited significant improvement in glycemic control in Goto-Kakizaki rats [23,24]. Although DJB was designed to assess the contribution of the upper small intestine to improvements in glucose homeostasis after RYGB [23,24], exendin9-39, a GLP-1 receptor antagonist, abolished the glucose-reducing effect of DJB [25]. This finding suggests that GLP-1, a representative hindgut hormone, is critical in mediating the glucose reduction resulting from DJB. Interestingly, it was reported that the number of K/L cells, which produce both GIP and GLP-1, was increased in the jejunum attached to the stomach in Goto-Kakizaki rats after DJB [26], indicating that GLP-1 secreted from this proximal intestinal segment may improve glucose homeostasis. Although GLP-1, a typical hindgut hormone, is important in improved glucose homeostasis after DJB, proteins obtained from the duodenum of db/db mice or insulin-resistant humans trigger insulin resistance both in vitro and in vivo [27]. Therefore, the so-called foregut factor (also known as anti-incretin) may contribute to the pathophysiology of type 2 diabetes. Interestingly, DJB or intrajejunal nutrient administration suppresses endogenous glucose production through the gut-brain-liver axis, presumably by stimulating the jejunal nutrient sensor [28]. Therefore, the mechanism of action of DJB is very complex. Aside from the debate whether the foregut factor or the hindgut factor is the major player in the improved glucose homeostasis after DJB, an endoluminal liner bypassing the duodenum and proximal jejunum showed promising results in body weight control and glucose metabolism in obese patients with type 2 diabetes [29].
- RYGB accelerates gastrointestinal transit of ingested food and thereby stimulates L-cell secretion of GLP-1, PYY, and oxyntomodulin, which regulates energy and glucose metabolism [30]. In patients with type 2 diabetes, RYGB increased plasma levels of GLP-1, PYY, and oxyntomodulin after oral glucose load, whereas calorie restriction alone did not [31]. Just as in RYGB, plasma GLP-1 levels markedly increased after BPD [32], which also expedites the transit of orally ingested foods to the distal intestine. However, the AGB procedure, which simply restricts food intake without accelerating gastrointestinal transit, does not increase plasma GLP-1 levels [33]. Unexpectedly, SG, which had previously been considered as a mere restrictive bariatric procedure, induces a marked increase in plasma GLP-1 levels [34,35]. Accelerated gastrointestinal transit and altered gastrointestinal physiology may explain the drastic increase in plasma GLP-1 levels after SG [12]. Considering the mechanism of action of GLP-1 [36], GLP-1 could be a critical factor in weight loss and diabetes remission after RYGB. However, the administration of a GLP-1 receptor antagonist (exendin9-39) did not reveal a significant increase in blood glucose levels in patients who showed remission of type 2 diabetes after RYGB [37], which indicates that factors other than GLP-1 may play an important role in diabetes remission following RYGB. To examine the role of the hindgut in an isolated fashion, an experimental surgical procedure called ileal transposition (or interposition) was developed [38,39,40]. Ileal transposition increases plasma levels of GLP-1 and PYY by promoting early contact between ingested nutrients and the ileal tissue transposed to the proximal jejunum [39,40,41,42,43]. However, the overall effect of ileal transposition on glucose metabolism and body weight is typically modest [38,39,40,41,42,43]. Therefore, the hindgut effect after RYGB may partly explain its diabetes remission mechanism.
- Altered glucose metabolism of the intestine may partially explain the diabetes remission observed after RYGB. Increased aerobic glycolysis in the Roux limb was observed in rats treated with RYGB [44], which allows this segment of the intestine to play a major role in glucose utilization. Consistent with this finding, one of the antidiabetic mechanisms of metformin is to induce a marked increase in intestinal glucose utilization [45]. Just like the liver and the kidneys, the intestine is able to produce glucose via gluconeogenesis. Interestingly, increased intestinal gluconeogenesis, particularly in the ileum, was seen after enterogastro-anastomosis (EGA), which does not create the Roux limb, in C57Bl/6 mice fed a high-fat diet. Increased gluconeogenesis in this model was accompanied by reduced food intake, increased insulin sensitivity, and decreased endogenous glucose production [46]. It was hypothesized that the increased glucose concentration in the portal vein may explain the beneficial effects of EGA. In this process, GLUT2 appears to be essential because GLUT2 knockout mice were not subject to the metabolic effects of EGA [46]. In addition, portal vein denervation also abolished the metabolic effects of EGA [46]. Therefore, altered glucose metabolism in the intestine, either through increased glucose utilization or increased gluconeogenesis, may contribute to improved systemic glucose metabolism after bariatric surgery.
- Pancreatic β-cell function
- Pancreatic β-cell function is improved in obese patients with type 2 diabetes in accordance with exaggerated GLP-1 secretion immediately after RYGB and SG [47]. However, the extent of recovery of β-cell glucose sensitivity (as measured by insulin secretion rates in response to increasing plasma glucose levels) usually falls short of the normal value [48], and baseline β-cell glucose sensitivity was reported to be a major determinant of diabetes remission after RYGB [47,49]. Antagonism of GLP-1 signaling by exendin9-39 decreases postprandial insulin secretion in these patients [50], which indicates that the restored β-cell function is largely mediated by the exaggerated GLP-1 response. Unlike the insulin response to oral glucose, the insulin response to intravenous glucose was unchanged relative to the preoperative value [51], which also highlights the importance of gastrointestinal factors in improving β-cell function. However, the disposition index (a measure of insulin secretory capacity considering the insulin sensitivity of an individual) during the frequently sampled intravenous glucose tolerance test increased mainly due to increased insulin sensitivity [52]. Although improved β-cell function after RYGB is largely ascribed to increased GLP-1 secretion, administration of exendin9-39 hardly deteriorated postprandial hyperglycemia in patients who showed improved postprandial glycemia after RYGB [37]. Therefore, factors other than GLP-1 play a major role in improved glucose homeostasis after RYGB.
- Hepatic and peripheral insulin sensitivity
- Changes in insulin sensitivity after RYGB vary among different tissues. Marked improvement in hepatic insulin sensitivity was observed as early as 1 week after RYGB and persisted for up to 1 year, while the insulin sensitivity of peripheral tissues, including skeletal muscle and adipose tissue, was not changed during the early postoperative period but improved gradually thereafter [51]. Improved hepatic insulin sensitivity is crucial in normal glucose homeostasis because it leads to decreased hepatic glucose production. Of note, hepatic insulin sensitivity dramatically improves even before any significant weight reduction occurs [51]. In this process, calorie restriction from the early postoperative period may play a critical role [53]. Although the exact mechanism of improved hepatic insulin sensitivity after RYGB is still elusive, decreased intrahepatic fat content may be responsible [54]. In a study using the euglycemic-hyperinsulinemic clamp, an improvement in peripheral insulin sensitivity after RYGB was observed only after a substantial weight loss [55]. In this regard, there appear to be many potential mechanisms for improved insulin sensitivity after RYGB [56]. Among these, weight loss-associated mechanisms are essential because improved insulin sensitivity is closely correlated with the degree of weight loss [47,51]. To summarize, hepatic insulin sensitivity improves immediately after RYGB and is largely explained by calorie restriction, while peripheral insulin sensitivity gradually improves in accordance with weight loss.
- Altered bile acid metabolism
- Plasma levels of bile acids are increased after RYGB [57] or SG [58], which prompted researchers to hypothesize that bile acids play a role in meditating the effects of bariatric surgery. Bile acids are known as detergents for fat absorption and routes of cholesterol elimination; however, many genomic and nongenomic actions largely related to metabolism have also been reported for this class of molecules [59]. In this regard, the nuclear receptor FXR and a G-protein coupled receptor TGR5 are known to mediate the genomic and non-genomic effects of bile acids, respectively [60]. In the absence of FXR signaling in mice, the beneficial effects of SG on body weight and glucose metabolism were abolished [61]. Further studies are needed to elucidate the exact mechanism of bile acids in weight control and glucose metabolism.
- Changes in gut microbiota
- The role of gut microbiota is currently discussed in terms of host-microbial interactions modulating host metabolism [62]. After bariatric surgery, a substantial change in gut microbiota has been reported not only in rodents but also in humans. In one study including humans, rats, and mice, RYGB induced rapid increases in the proportion of Gammaproteobacteria (Escherichia) and Verrucomicrobia (Akkermansia) in the gut [63]. Interestingly, a recent study showed that metformin, a representative antidiabetes medication, increased the relative abundance of Akkermansia muciniphila in mice [64]. However, whether metformin and RYGB similarly affect gut microbiota and thus improve glucose homeostasis is uncertain. When gut microbiota from mice subjected to RYGB were transferred to nonoperated germ-free mice, weight loss and decreased fat mass were observed [63]. However, transfer of gut microbiota from sham-operated mice to germ-free mice did not induce substantial weight loss [63]. The mechanism of weight loss via altered host-microbial interactions after RYGB is still elusive, but changes in the production of short-chain fatty acids by gut microbiota may play a role [63]. Although the causal relationship between altered gut microbiota and improved glucose control after RYGB is uncertain in humans, it is very interesting that fecal transplant from lean donors to metabolically unhealthy people improved insulin sensitivity and increased populations of butyrate-producing gut microbiota [65]. Further studies are mandatory to exploit the mechanisms of altered host-microbial interactions for the treatment of diabetes and obesity in humans.
POTENTIAL MECHANISMS OF DIABETES REMISSION FOLLOWING BARIATRIC SURGERY
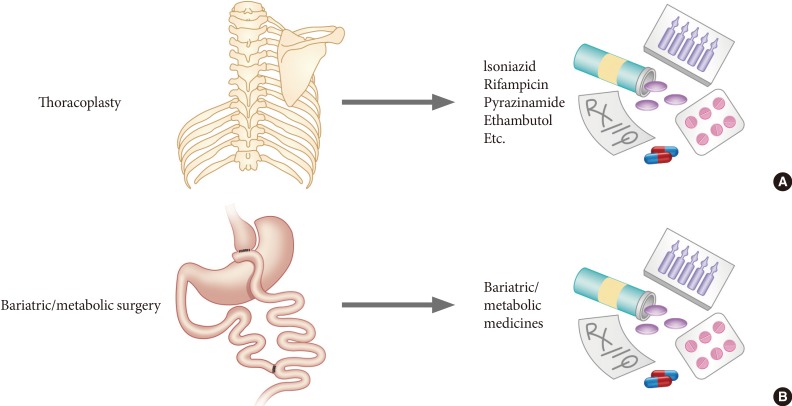
- The current obesity and type 2 diabetes epidemics may only be halted by breakthrough knowledge, as conventional treatments have thus far proven unsuccessful. The dramatic improvement in glucose metabolism observed following RYGB and SG prompted "gut feelings" regarding a cure for diabetes. However, such surgical procedures are not always simple and safe but sometimes cause considerable morbidity and mortality, although the incidence of complications is low. For some patients and doctors, medical treatment is preferred to surgical treatment. By elucidating the mechanisms of diabetes remission after RYGB and SG, we may be able to develop both efficacious and safe medical treatments for diabetes and/or obesity. However, our understanding of the mechanisms of diabetes remission following bariatric surgery is still very limited, although the body of knowledge is rapidly expanding (Fig. 2). It is pertinent to recall the history of antituberculosis treatment [66]. Once, thoracoplasty, which removes the ribs and collapses the diseased lung, was widely used to treat pulmonary tuberculosis but was abandoned after development of effective antimycobacterial pharmacotherapy. It is our hope that bariatric surgery may be replaced by medical therapy, just as in the case of thoracoplasty (Fig. 3).
CONCLUSIONS
- 1. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM, Dohm L. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339-350. ArticlePubMedPMC
- 2. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724-1737. ArticlePubMed
- 3. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577-1585. ArticlePubMed
- 4. Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567-1576. ArticlePubMedPMC
- 5. Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H. Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683-2693. ArticlePubMed
- 6. Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM. Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741-752. ArticlePubMed
- 7. Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, Ahlin S, Anveden A, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lonroth H, Narbro K, Naslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56-65. ArticlePubMed
- 8. Sjostrom L, Gummesson A, Sjostrom CD, Narbro K, Peltonen M, Wedel H, Bengtsson C, Bouchard C, Carlsson B, Dahlgren S, Jacobson P, Karason K, Karlsson J, Larsson B, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Carlsson LM. Swedish Obese Subjects Study. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol 2009;10:653-662. ArticlePubMed
- 9. Carlsson LM, Peltonen M, Ahlin S, Anveden A, Bouchard C, Carlsson B, Jacobson P, Lonroth H, Maglio C, Naslund I, Pirazzi C, Romeo S, Sjoholm K, Sjostrom E, Wedel H, Svensson PA, Sjostrom L. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695-704. ArticlePubMed
- 10. Sjostrom L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden A, Bouchard C, Carlsson B, Karason K, Lonroth H, Naslund I, Sjostrom E, Taube M, Wedel H, Svensson PA, Sjoholm K, Carlsson LM. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297-2304. ArticlePubMed
- 11. Karlsson J, Taft C, Ryden A, Sjostrom L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes (Lond) 2007;31:1248-1261. ArticlePubMedPDF
- 12. Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, Greer T, Sorrell J, Lemmen L, LaSance K, Woods SC, Seeley RJ, D'Alessio DA, Sandoval DA. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab 2014;306:E424-E432. ArticlePubMed
- 13. Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab 2004;89:2608-2615. ArticlePubMed
- 14. Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 2005;15:474-481. ArticlePubMedPDF
- 15. Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316-323. ArticlePubMed
- 16. Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, Marks-Shulman PA, Abumrad NN. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 2010;33:1438-1442. ArticlePubMedPMCPDF
- 17. Lingvay I, Guth E, Islam A, Livingston E. Rapid improvement in diabetes after gastric bypass surgery: is it the diet or surgery? Diabetes Care 2013;36:2741-2747. PubMedPMC
- 18. Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, Gastaldelli A, Chambers KT, Su X, Okunade A, Patterson BW, Klein S. Gastric bypass and banding equally improve insulin sensitivity and beta cell function. J Clin Invest 2012;122:4667-4674. ArticlePubMedPMC
- 19. Dirksen C, Jorgensen NB, Bojsen-Moller KN, Jacobsen SH, Hansen DL, Worm D, Holst JJ, Madsbad S. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia 2012;55:1890-1901. ArticlePubMedPDF
- 20. Thaler JP, Cummings DE. Minireview: hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 2009;150:2518-2525. ArticlePubMed
- 21. Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, Kern B, von Fluee M, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg 2012;22:740-748. ArticlePubMedPMC
- 22. Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschop MH, Sandoval DA, Seeley RJ. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology 2013;144:50-52. ArticlePubMed
- 23. Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006;244:741-749. ArticlePubMedPMC
- 24. Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004;239:1-11. PubMedPMC
- 25. Kindel TL, Yoder SM, Seeley RJ, D'Alessio DA, Tso P. Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg 2009;13:1762-1772. ArticlePubMedPDF
- 26. Speck M, Cho YM, Asadi A, Rubino F, Kieffer TJ. Duodenal-jejunal bypass protects GK rats from {beta}-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. Am J Physiol Endocrinol Metab 2011;300:E923-E932. PubMed
- 27. Salinari S, Debard C, Bertuzzi A, Durand C, Zimmet P, Vidal H, Mingrone G. Jejunal proteins secreted by db/db mice or insulin-resistant humans impair the insulin signaling and determine insulin resistance. PLoS One 2013;8:e56258ArticlePubMedPMC
- 28. Breen DM, Rasmussen BA, Kokorovic A, Wang R, Cheung GW, Lam TK. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med 2012;18:950-955. ArticlePubMedPDF
- 29. Koehestanie P, de Jonge C, Berends FJ, Janssen IM, Bouvy ND, Greve JW. The effect of the endoscopic duodenal-jejunal bypass liner on obesity and type 2 diabetes mellitus, a multicenter randomized controlled trial. Ann Surg 2014 7 28 Epub. DOI: http://dx.doi.org/10.1097/SLA.0000000000000794.Article
- 30. Cho YM, Merchant CE, Kieffer TJ. Targeting the glucagon receptor family for diabetes and obesity therapy. Pharmacol Ther 2012;135:247-278. ArticlePubMed
- 31. Laferrere B, Swerdlow N, Bawa B, Arias S, Bose M, Olivan B, Teixeira J, McGinty J, Rother KI. Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab 2010;95:4072-4076. ArticlePubMedPMC
- 32. Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care 2009;32:375-380. ArticlePubMedPMCPDF
- 33. Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, Kirwan JP, Schauer PR. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2010;34:462-471. PubMed
- 34. Jimenez A, Casamitjana R, Flores L, Viaplana J, Corcelles R, Lacy A, Vidal J. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg 2012;256:1023-1029. ArticlePubMed
- 35. Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, Vidal J. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc 2012;26:2231-2239. ArticlePubMedPDF
- 36. Cho YM, Fujita Y, Kieffer TJ. Glucagon-like peptide-1: glucose homeostasis and beyond. Annu Rev Physiol 2014;76:535-559. ArticlePubMed
- 37. Jimenez A, Casamitjana R, Viaplana-Masclans J, Lacy A, Vidal J. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care 2013;36:2062-2069. ArticlePubMedPMCPDF
- 38. Patriti A, Aisa MC, Annetti C, Sidoni A, Galli F, Ferri I, Gulla N, Donini A. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery 2007;142:74-85. ArticlePubMed
- 39. Strader AD, Vahl TP, Jandacek RJ, Woods SC, D'Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 2005;288:E447-E453. ArticlePubMed
- 40. Wang TT, Hu SY, Gao HD, Zhang GY, Liu CZ, Feng JB, Frezza EE. Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann Surg 2008;247:968-975. ArticlePubMed
- 41. Culnan DM, Albaugh V, Sun M, Lynch CJ, Lang CH, Cooney RN. Ileal interposition improves glucose tolerance and insulin sensitivity in the obese Zucker rat. Am J Physiol Gastrointest Liver Physiol 2010;299:G751-G760. ArticlePubMedPMC
- 42. Ikezawa F, Shibata C, Kikuchi D, Imoto H, Miura K, Naitoh T, Ogawa H, Sasaki I, Tsuchiya T. Effects of ileal interposition on glucose metabolism in obese rats with diabetes. Surgery 2012;151:822-830. ArticlePubMed
- 43. Strader AD. Ileal transposition provides insight into the effectiveness of gastric bypass surgery. Physiol Behav 2006;88:277-282. ArticlePubMed
- 44. Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 2013;341:406-410. ArticlePubMedPMC
- 45. Gontier E, Fourme E, Wartski M, Blondet C, Bonardel G, Le Stanc E, Mantzarides M, Foehrenbach H, Pecking AP, Alberini JL. High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol Imaging 2008;35:95-99. ArticlePubMedPDF
- 46. Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X, Pillot B, Fauveau V, Aubert R, Viollet B, Foretz M, Leclerc J, Duchampt A, Zitoun C, Thorens B, Magnan C, Mithieux G, Andreelli F. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 2008;8:201-211. ArticlePubMed
- 47. Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, Barsotti E, Berta R, Moriconi D, Bellini R, Anselmino M, Ferrannini E. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab 2013;98:4391-4399. ArticlePubMed
- 48. Camastra S, Muscelli E, Gastaldelli A, Holst JJ, Astiarraga B, Baldi S, Nannipieri M, Ciociaro D, Anselmino M, Mari A, Ferrannini E. Long-term effects of bariatric surgery on meal disposal and beta-cell function in diabetic and nondiabetic patients. Diabetes 2013;62:3709-3717. PubMedPMC
- 49. Nannipieri M, Mari A, Anselmino M, Baldi S, Barsotti E, Guarino D, Camastra S, Bellini R, Berta RD, Ferrannini E. The role of beta-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J Clin Endocrinol Metab 2011;96:E1372-E1379. PubMed
- 50. Salehi M, Prigeon RL, D'Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011;60:2308-2314. ArticlePubMedPMCPDF
- 51. Bojsen-Moller KN, Dirksen C, Jorgensen NB, Jacobsen SH, Serup AK, Albers PH, Hansen DL, Worm D, Naver L, Kristiansen VB, Wojtaszewski JF, Kiens B, Holst JJ, Richter EA, Madsbad S. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes 2014;63:1725-1737. ArticlePubMedPDF
- 52. Lin E, Liang Z, Frediani J, Davis SS Jr, Sweeney JF, Ziegler TR, Phillips LS, Gletsu-Miller N. Improvement in ss-cell function in patients with normal and hyperglycemia following Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab 2010;299:E706-E712. PubMedPMC
- 53. Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 2009;136:1552-1560. ArticlePubMedPMC
- 54. Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506-2514. ArticlePubMedPMC
- 55. Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM, Havel P, Schambelan M, Mulligan K. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg 2010;14:15-23. ArticlePubMed
- 56. Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol 2014;2:152-164. ArticlePubMed
- 57. Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671-1677. ArticlePubMedPMCPDF
- 58. Stefater MA, Sandoval DA, Chambers AP, Wilson-Perez HE, Hofmann SM, Jandacek R, Tso P, Woods SC, Seeley RJ. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology 2011;141:939-949. ArticlePubMedPMC
- 59. Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap: bile acids in metabolic control. Nat Rev Endocrinol 2014;10:488-498. ArticlePubMedPDF
- 60. de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab 2013;17:657-669. ArticlePubMedPMC
- 61. Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Perez HE, Sandoval DA, Kohli R, Backhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014;509:183-188. ArticlePubMedPMCPDF
- 62. Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science 2012;336:1262-1267. ArticlePubMed
- 63. Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 2013;5:178ra41.ArticlePubMedPMC
- 64. Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014;63:727-735. ArticlePubMed
- 65. Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012;143:913-916. ArticlePubMed
- 66. Daniel TM. The history of tuberculosis. Respir Med 2006;100:1862-1870. ArticlePubMed
REFERENCES
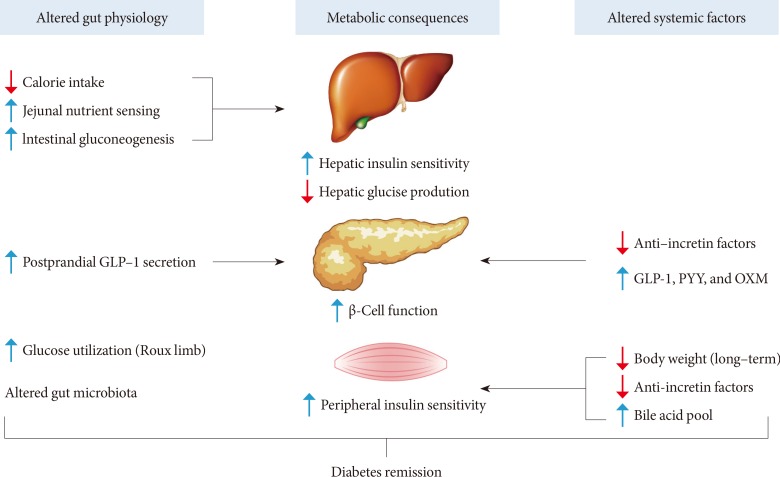
Fig. 2Potential mechanisms of diabetes remission after Roux-en-Y gastric bypass. Altered gut physiology and systemic (or circulating) factors contribute to improved metabolic states in concert after Roux-en-Y gastric bypass. See text for detailed explanation. GLP-1, glucagon-like peptide-1; PYY, peptide-YY; OXM, oxyntomodulin.


Figure & Data
References
Citations
Citations to this article as recorded by 

- The Effect of Diet Composition on the Post-operative Outcomes of Roux-en-Y Gastric Bypass in Mice
Matthew Stevenson, Ankita Srivastava, Maria Nacher, Christopher Hall, Thomas Palaia, Jenny Lee, Chaohui Lisa Zhao, Raymond Lau, Mohamed A. E. Ali, Christopher Y. Park, Florencia Schlamp, Sean P. Heffron, Edward A. Fisher, Collin Brathwaite, Louis Ragolia
Obesity Surgery.2024; 34(3): 911. CrossRef - A Matched Comparative Analysis of Type-2 Diabetes Mellitus Remission Between Roux-en-Y Gastric Bypass and Sleeve Gastrectomy
Karl Hage, Pearl Ma, Wissam Ghusn, Kayla Ikemiya, Andres Acosta, Robert A. Vierkant, Barham K. Abu Dayyeh, Kelvin D. Higa, Omar M. Ghanem
Surgical Innovation.2024; 31(2): 148. CrossRef - Sex-Specific Changes in Body Composition Following Metabolic and Bariatric Surgery Are Associated with the Remission of Metabolic Syndrome
Xianhao Yi, Weizheng Li, Guohui Wang, Pengzhou Li, Xulong Sun, Haibo Tang, Beibei Cui, Jiapu Ling, Ping Luo, Zhibing Fu, Hui Zhou, Liyong Zhu, Shaihong Zhu
Obesity Surgery.2023; 33(9): 2780. CrossRef - East Asian perspectives in metabolic and bariatric surgery
Tae Jung Oh, Hyuk‐Joon Lee, Young Min Cho
Journal of Diabetes Investigation.2022; 13(5): 756. CrossRef - Long-Term Trajectories in Weight and Health Outcomes Following Multidisciplinary Publicly Funded Bariatric Surgery in Patients with Clinically Severe Obesity (≥ 3 Associated Comorbidities): A Nine-Year Prospective Cohort Study in Australia
Michelle M.C. Tan, Xingzhong Jin, Craig Taylor, Adrian K. Low, Philip Le Page, David Martin, Ang Li, David Joseph, Nic Kormas
Journal of Clinical Medicine.2022; 11(15): 4466. CrossRef - Impact of Bariatric Surgery in Reducing Macrovascular Complications in Severely Obese T2DM Patients
Salman Hussain, Mohd Shahnawaz Khan, Mohammad Chand Jamali, Ali Nasir Siddiqui, Gaurav Gupta, Md Sarfaraj Hussain, Fohad Mabood Husain
Obesity Surgery.2021; 31(5): 1929. CrossRef - Vertical sleeve gastrectomy induces distinctive transcriptomic responses in liver, fat and muscle
Chang Ho Ahn, Eun Hye Choi, Hyunjung Lee, Woochan Lee, Jong-Il Kim, Young Min Cho
Scientific Reports.2021;[Epub] CrossRef - Which predictors could effect on remission of type 2 diabetes mellitus after the metabolic surgery: A general perspective of current studies?
Gamze Akkus, Tamer Tetiker
World Journal of Diabetes.2021; 12(8): 1312. CrossRef - Impact of Metabolic Surgery on Type-2 Diabetes Remission
Cejana de Abrantes Figueiredo Baiocchi, Diana Aristótelis Rocha de Sá
Current Diabetes Reviews.2021;[Epub] CrossRef - Ileal Transposition Increases Pancreatic β Cell Mass and Decreases β Cell Senescence in Diet-Induced Obese Rats
Chang Ho Ahn, Eun Hye Choi, Tae Jung Oh, Young Min Cho
Obesity Surgery.2020; 30(5): 1849. CrossRef - Does Reconstruction Type After Gastric Resection Matters for Type 2 Diabetes Improvement?
Mariana Costa, Artur Trovão Lima, Tiago Morais, Rui F. Almeida, Mário Nora, Marta Guimarães, Mariana P. Monteiro
Journal of Gastrointestinal Surgery.2020; 24(6): 1269. CrossRef - Epidemiology, pathophysiology and etiology of obesity in children and adolescents
Jessica Kerns, Martin Fisher
Current Problems in Pediatric and Adolescent Health Care.2020; 50(9): 100869. CrossRef - Obesity in Adolescents and Youth: The Case for and against Bariatric Surgery
Ahmed Khattab, Mark A. Sperling
The Journal of Pediatrics.2019; 207: 18. CrossRef - Outcomes of Bariatric Surgery Versus Medical Management for Type 2 Diabetes Mellitus: a Meta-Analysis of Randomized Controlled Trials
Zhamak Khorgami, Saeed Shoar, Alan A. Saber, C. Anthony Howard, Goodarz Danaei, Guido M. Sclabas
Obesity Surgery.2019; 29(3): 964. CrossRef - Validating Risk Prediction Models of Diabetes Remission After Sleeve Gastrectomy
Shih-Chiang Shen, Weu Wang, Ka-Wai Tam, Hsin-An Chen, Yen-Kuang Lin, Shih-Yun Wang, Ming-Te Huang, Yen-Hao Su
Obesity Surgery.2019; 29(1): 221. CrossRef - Long-term diabetes outcomes after bariatric surgery—managing medication withdrawl
Pedro Souteiro, Sandra Belo, Daniela Magalhães, Jorge Pedro, João Sérgio Neves, Sofia Castro Oliveira, Paula Freitas, Ana Varela, Davide Carvalho
International Journal of Obesity.2019; 43(11): 2217. CrossRef - What is type 2 diabetes?
Maria Daniela Hurtado, Adrian Vella
Medicine.2019; 47(1): 10. CrossRef - Intestinal Glucose Absorption Was Reduced by Vertical Sleeve Gastrectomy via Decreased Gastric Leptin Secretion
Jinpeng Du, Chaojie Hu, Jie Bai, Miaomiao Peng, Qingbo Wang, Ning Zhao, Yu Wang, Guobin Wang, Kaixiong Tao, Geng Wang, Zefeng Xia
Obesity Surgery.2018; 28(12): 3851. CrossRef - Non-pharmacological Treatment Options in the Management of Diabetes Mellitus
Arkiath V Raveendran
European Endocrinology.2018; 14(2): 31. CrossRef - Ileal transposition rapidly improves glucose tolerance and gradually improves insulin resistance in non-obese type 2 diabetic rats
Hengliang Zhu, Huaiming Wang, Zhihai Zheng, Bailiang Ye, Xiaojiao Ruan, Xiaofeng Zheng, Guoxin Li
Gastroenterology Report.2018; 6(4): 291. CrossRef - Small Intestinal Bypass Induces a Persistent Weight-Loss Effect and Improves Glucose Tolerance in Obese Rats
Jiaqing Cao, Quan Ren, Cai Tan, Jinyuan Duan
Obesity Surgery.2017; 27(7): 1859. CrossRef - Diabetes improvement and resolution following laparoscopic vertical sleeve gastrectomy (LVSG) versus laparoscopic Roux-en-Y gastric bypass (LRYGB) procedures: a systematic review of randomized controlled trials
Emma Osland, Rossita Mohamad Yunus, Shahjahan Khan, Breda Memon, Muhammed Ashraf Memon
Surgical Endoscopy.2017; 31(4): 1952. CrossRef - The Long-Term Effects of Bariatric Surgery on Type 2 Diabetes Remission, Microvascular and Macrovascular Complications, and Mortality: a Systematic Review and Meta-Analysis
Binwu Sheng, Khoa Truong, Hugh Spitler, Lu Zhang, Xuetao Tong, Liwei Chen
Obesity Surgery.2017; 27(10): 2724. CrossRef - Long-term effects of duodenojejunal bypass on diabetes in Otsuka Long–Evans Tokushima Fatty rats
Sang Kuon Lee, Oh-Joo Kwon, Hae Myung Jeon, Say-June Kim
Asian Journal of Surgery.2017; 40(4): 262. CrossRef - Interaction Between Atypical Antipsychotics and the Gut Microbiome in a Bipolar Disease Cohort
Stephanie A. Flowers, Simon J. Evans, Kristen M. Ward, Melvin G. McInnis, Vicki L. Ellingrod
Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy.2017; 37(3): 261. CrossRef - THE ROLE OF THE SLEEVE GASTRECTOMY AND THE MANAGEMENT OF TYPE 2 DIABETES
Taíse FUCHS, Marcelo LOUREIRO, Gabriela Heloise BOTH, Heloise Helena SKRABA, Thaís Andrade COSTA-CASAGRANDE
ABCD. Arquivos Brasileiros de Cirurgia Digestiva (São Paulo).2017; 30(4): 283. CrossRef - Attenuated secretion of glucose-dependent insulinotropic polypeptide (GIP) does not alleviate hyperphagic obesity and insulin resistance in ob/ob mice
Satoko Shimazu-Kuwahara, Norio Harada, Shunsuke Yamane, Erina Joo, Akiko Sankoda, Timothy J. Kieffer, Nobuya Inagaki
Molecular Metabolism.2017; 6(3): 288. CrossRef - Adipose tissue supports normalization of macrophage and liver lipid handling in obesity reversal
Maayan Vatarescu, Sapir Bechor, Yulia Haim, Tal Pecht, Tanya Tarnovscki, Noa Slutsky, Ori Nov, Hagit Shapiro, Avishai Shemesh, Angel Porgador, Nava Bashan, Assaf Rudich
Journal of Endocrinology.2017; 233(3): 293. CrossRef - Sleeve Gastrectomy Alters Intestinal Permeability in Diet-Induced Obese Mice
Claire Blanchard, François Moreau, Julien Chevalier, Audrey Ayer, Damien Garcon, Lucie Arnaud, Jean-Paul Pais de Barros, Thomas Gautier, Michel Neunlist, Bertrand Cariou, Cédric Le May
Obesity Surgery.2017; 27(10): 2590. CrossRef - Long-term Follow-up for Type 2 Diabetes Mellitus after Gastrectomy in Non-morbidly Obese Patients with Gastric Cancer: the Legitimacy of Onco-metabolic Surgery
Tae-Hoon Lee, Chang Min Lee, Sungsoo Park, Do Hyun Jung, You Jin Jang, Jong-Han Kim, Seong-Heum Park, Young-Jae Mok
Journal of Gastric Cancer.2017; 17(4): 283. CrossRef - Preoperative Beta Cell Function Is Predictive of Diabetes Remission After Bariatric Surgery
Pedro Souteiro, Sandra Belo, João Sérgio Neves, Daniela Magalhães, Rita Bettencourt Silva, Sofia Castro Oliveira, Maria Manuel Costa, Ana Saavedra, Joana Oliveira, Filipe Cunha, Eva Lau, César Esteves, Paula Freitas, Ana Varela, Joana Queirós, Davide Carv
Obesity Surgery.2017; 27(2): 288. CrossRef - Ileal Transposition Decreases Plasma Lipopolysaccharide Levels in Association with Increased L Cell Secretion in Non-obese Non-diabetic Rats
Tae Jung Oh, Hyuk-Joon Lee, Young Min Cho
Obesity Surgery.2016; 26(6): 1287. CrossRef - The Mechanism of Metabolic Surgery: Gastric Center Hypothesis
Jiangfan Zhu, Radheshyam Gupta, Mahmood Safwa
Obesity Surgery.2016; 26(7): 1639. CrossRef - Lipids and bariatric procedures Part 2 of 2: scientific statement from the American Society for Metabolic and Bariatric Surgery (ASMBS), the National Lipid Association (NLA), and Obesity Medicine Association (OMA)
Harold Bays, Shanu N. Kothari, Dan E. Azagury, John M. Morton, Ninh T. Nguyen, Peter H. Jones, Terry A. Jacobson, David E. Cohen, Carl Orringer, Eric C. Westman, Deborah B. Horn, Wendy Scinta, Craig Primack
Surgery for Obesity and Related Diseases.2016; 12(3): 468. CrossRef - Metabolic Surgery for Type 2 Diabetes Mellitus: Experience from Asia
Wei-Jei Lee, Lwin Aung
Diabetes & Metabolism Journal.2016; 40(6): 433. CrossRef - Improved glucose metabolism following bariatric surgery is associated with increased circulating bile acid concentrations and remodeling of the gut microbiome
Lukasz Kaska, Tomasz Sledzinski, Agnieszka Chomiczewska, Agnieszka Dettlaff-Pokora, Julian Swierczynski
World Journal of Gastroenterology.2016; 22(39): 8698. CrossRef - In Vivo Models for Incretin Research: From the Intestine to the Whole Body
Tae Jung Oh
Endocrinology and Metabolism.2016; 31(1): 45. CrossRef - Contribution of the distal small intestine to metabolic improvement after bariatric/metabolic surgery: Lessons from ileal transposition surgery
Tae Jung Oh, Chang Ho Ahn, Young Min Cho
Journal of Diabetes Investigation.2016; 7(S1): 94. CrossRef - EFFECTS OF LONG-TERM ROUX-EN-Y GASTRIC BYPASS ON BODY WEIGHT AND CLINICAL METABOLIC COMORBIDITIES IN BARIATRIC SURGERY SERVICE OF A UNIVERSITY HOSPITAL
Cátia Ferreira da SILVA, Larissa COHEN, Luciana d'Abreu SARMENTO, Felipe Monnerat Marino ROSA, Eliane Lopes ROSADO, João Régis Ivar CARNEIRO, Antônio Augusto Peixoto de SOUZA, Fernanda Cristina Carvalho Mattos MAGNO
ABCD. Arquivos Brasileiros de Cirurgia Digestiva (São Paulo).2016; 29( suppl 1): 20. CrossRef - Medication Use Among Patients Prior to Bariatric Surgery
Jennifer Padden Elliott, Erica L. Gray, Jessie Yu, Melissa A. Kalarchian
Bariatric Surgical Practice and Patient Care.2015; 10(3): 105. CrossRef - Changes in the salivary protein profile of morbidly obese women either previously subjected to bariatric surgery or not
Elsa Lamy, Carla Simões, Lénia Rodrigues, Ana Rodrigues Costa, Rui Vitorino, Francisco Amado, Célia Antunes, Isabel do Carmo
Journal of Physiology and Biochemistry.2015; 71(4): 691. CrossRef

 KDA
KDA
 PubReader
PubReader Cite
Cite